To determine if hyperintense fluid in the postsurgical cavity on follow-up fluid-attenuated inversion recovery (FLAIR) sequences can predict progression in gliomas.
Material and MethodsObservational study of magnetic resonance imaging signal of fluid within the post-surgical cavity in patients with glioma (grade II–IV), with surgery and follow-up between 2007 and 2012. Qualitative comparison between the signal of fluid in the cavity and of the ventricular cerebrospinal fluid (CSF) was performed on FLAIR sequences. Fluid in the cavity was classified as isointense or hyperintense compared to CSF. Double-blind reading was performed. The signal intensity was correlated with tumour progression, assessed using Response Assessment in Neuro-Oncology criteria.
ResultsA total of 107 patients were included, of whom 90 had high-grade gliomas. Inter-rater agreement was excellent, and intra-rater complete (k=0.94 and 1, p<.001). Hyperintense fluid in the resection cavity occurred more commonly (58.9% versus 29.4%, p=.025) and earlier (mean 4.5 versus 9.9 months, p<.001) in high-grade than in low-grade gliomas. Hyperintense fluid was associated with progression in high-grade gliomas, with a sensitivity of 65.7% (95%CI, 54.3–75.6%) and a specificity of 70.6% (95%CI, 46.6–87%), and in low-grade gliomas with a sensitivity of 50% (95%CI, 18.7–81.2%), and a specificity of 81.8% (95%CI, 51.1–96%). The positive predictive value of this sign was 90.6% (95%CI, 79.3–96.3%) for high-grade gliomas, and was higher for grade IV (93.2%, 95%CI, 87.3–99.1%) and lower for grade III (77.8%, 95%CI, 59.6–96%), and low-grade gliomas (60%, 95%CI, 22.9–88.4%). False-positives were identified in 7 patients, due to bleeding or infection. Hyperintense fluid in high-grade gliomas preceded progression in 22 patients (30.1%), with a mean of 4.1 months (SD 2.1, 95% CI, 3.2–5), and associated with poorer progression-free survival (mean 6.8 versus 11.7 months, p=.004).
ConclusionsHyperintense fluid in the resection cavity on follow-up FLAIR sequences occurs more frequently and earlier in high-grade gliomas, and is associated with poorer progression-free survival. Hyperintense fluid is associated with disease progression, and can predict the progression of resected gliomas. False-positives due to bleeding and infection can be observed, and are easily recognizable.
Analizar si la hiperseñal en la cavidad posquirúrgica en secuencia FLAIR puede predecir la progresión en gliomas.
Material y métodosEstudio observacional de la señal en resonancia magnética de la cavidad posquirúrgica en pacientes con glioma (gradoii-iv), con cirugía y seguimiento entre 2007 y 2012. La comparación cualitativa entre la señal del líquido en la cavidad y del líquido cefalorraquídeo (LCR) normal se realizó en las secuencias FLAIR. El líquido en la cavidad se clasificó como isointenso o hiperintenso en comparación con el LCR. Se utilizó una lectura doble ciego. La intensidad de la señal se correlacionó con la progresión tumoral evaluada según los criterios RANO.
ResultadosSe incluyeron 107 pacientes, 90 con gliomas de alto grado. La correlación entre los lectores fue excelente, y la intralector, completa (k=0,94 y 1, p<0,001). La hiperseñal en la cavidad de resección ocurrió con mayor frecuencia (58,9% versus 29,4%, p=0,025) y más temprano (media 4,5 frente a 9,9 meses, p<0,001) en los gliomas de alto grado que en los de bajo grado. La hiperseñal se asoció a la progresión en los gliomas de alto grado con una sensibilidad del 65,7% (IC95%, 54,3-75,6%) y una especificidad del 70,6% (IC95%, 46,6-87%), y en los gliomas de bajo grado con una sensibilidad del 50% (IC95%, 18,7-81,2%) y una especificidad del 81,8% (IC95%, 51,1-96%). El valor predictivo positivo de este signo fue del 90,6% (IC95%, 79,3-96,3%) para los gliomas de alto grado, más alto (93,2%, IC95%, 87,3-99,1%) para los de gradoiv y bajo (77,8%, IC95%, 59,6-96%) para los de gradoiii y para gliomas de bajo grado (60%, IC95%, 22,9-88,4%). En 7 pacientes se identificaron falsos positivos debidos a sangrado o infección. La hiperseñal en la cavidad en gliomas de alto grado precedió la progresión en 22 pacientes (30,1%), con una media de 4,1meses (DE 2,1, IC95%, 3,2-5), y se asoció a peor supervivencia libre de progresión (media 6,8 frente a 11,7meses, p=0,004).
ConclusionesLa hiperseñal en la cavidad de resección en secuencias FLAIR ocurre con más frecuencia y más temprano en gliomas de alto grado, y se asocia a peor supervivencia libre de progresión. La hiperseñal en la cavidad se asocia a la progresión de la enfermedad y puede predecir la progresión de los gliomas operados. Pueden darse falsos positivos debidos a hemorragia e infección, y son fácilmente reconocibles.
Glial tumours or gliomas can be classified as low-grade or high-grade tumours. Low-grade gliomas include grade I (circumscribed gliomas) and grade II (well-differentiated diffusely infiltrating gliomas). High-grade gliomas, also known as malignant gliomas, include grade III (anaplastyc gliomas) and grade IV (glioblastoma as the prototype).1 Malignant gliomas can occur “de novo” or can be the result of anaplastyc transformation of a grade II glioma with evolution to anaplastyc glioma, and finally glioblastoma (GB). GBs account for more than half of gliomas in adults, have a mean age of 64 years at diagnosis, and a mean survival of 12–15 months.2,3 Standard treatment is resection when possible, associated with radiation and chemotherapy.4,5 Despite outstanding recent advances in understanding the pathogenesis, imaging and treatment, the progression-free and overall survival in gliomas has remained poor over the last decades.3,6
Macdonald criteria were classically used to assess the treatment response for high-grade gliomas.7 They were based on measurements of the enhancing tumour, symptomatology, and the need for corticosteroids. Macdonald criteria have significant limitations including the necessity for characterization of the non-enhancing component of the tumour, and the non-specificity of enhancement with increasing incidence of pseudoprogression and pseudoresponse with the use of new therapies. These led to the updated recommendations by the Response Assessment in Neuro-Oncology (RANO) Working Group.8 A novelty of RANO criteria was the inclusion of fluid-attenuated inversion recovery (FLAIR) and T2-weighted images to assess the non-enhancing component of the tumour. Moreover, FLAIR seems to be useful not only for evaluation of the non-enhancing tumour, but also for assessment of the signal of fluid in the resection cavity on follow-up exams. In a previous study, consisting mainly of low-grade gliomas, a high-signal in the resection cavity on follow-up FLAIR sequences indicated progression with high specificity.9 A recent similar study in a small cohort of high-grade gliomas obtained similar data.10 These previous results have not yet been confirmed in larger series. Therefore, our study was designed primarily to check if hyperintense fluid in the postsurgical cavity on follow-up FLAIR images can predict progression in a large cohort of gliomas, and secondly, to seek further understanding of the practical utility of this finding.
MethodsThis unicentre observational cross-sectional study included the patients presenting with type II, III and IV brain gliomas (World Health Organization 2007) with curative intent surgery, histology diagnosis, and follow-up in our institution between 2007 and 2012. The local institutional review board approved the study. The patients were recruited from the database of the local Committee of Brain Tumours. The age, gender and histological type of tumour for each patient were registered. Due to their different behaviour, grade I gliomas were not included in the study. Exclusion criteria were: uncertain histological grade, no or only one follow-up magnetic resonance imaging (MRI), biopsy or partial excision instead of curative intent resection, absence of a resection cavity (or very small, defined as less than 15mm diameter), and intracavitary chemotherapy.
The MRI studies were acquired at MRI units of 1.5T and 3T, using a standard protocol which includes T1-weighted, T2-weighted, FLAIR images, diffusion (DWI), susceptibility-weighted (SWI) or gradient-recalled echo (GRE), contrast-enhanced T1-weighted, dynamic susceptibility contrast-enhanced (DSCE) perfusion images and spectroscopy. Follow-up studies were performed in the same unit at the same field-strength as was the preoperative study, and included a control MRI 1–4 days after surgery, 1 month after completing radiotherapy, and every 3 months. MRIs with significant artefacts were not included in the analysis. The number of follow-up MRI exams was calculated until progression, or until the last follow-up in patients without recurrence. The largest diameter of the resection cavity was registered.
A qualitative comparison between signal in the resection cavity and signal of ventricular cerebro-spinal fluid (CSF) was performed in follow-up FLAIR sequences. The signal in the cavity was defined as either isointense or hyperintense compared to the ventricular CSF. High-signal observed in the first 4 days following surgery, caused by debris and haemorrhages, and normalized in subsequent studies, was not considered as hyperintense in the analysis; normalization of the signal was considered by comparison to the normal signal of the ventricular CSF.
All MRI studies were read by two senior neuroradiologists blinded to the clinical data. Subsequently, a junior neuroradiologist (NS) performed an anonymized reading of the signal of fluid in the resection cavity in all follow-up FLAIR sequences of the 107 patients, and assessed whether the fluid was iso- or hyperintense compared to ventricular CSF. Double-blind reading of the signal of fluid in the resection cavity was used to assess inter-rater and intra-rater reliability; the last one was calculated by performing a second reading of 30 FLAIR sequences 4 months after the first reading; in the case of discrepancy, the decision was reached by consensus between readers.
RANO criteria were used to assess tumour progression. The progression-free survival was registered, as well as the mean time until hyperintense fluid occurred. The relation between hyperintense fluid in the cavity and tumour progression was analyzed. The analysis was carried out for all MRI scans acquired until progression was established, or until the last MRI in patients without progression. The analysis was performed separately for low-grade and high-grade gliomas; subsequently, the analyses of high-grade gliomas were subdivided in grade III and IV gliomas.
Statistical analysisA descriptive analysis was performed, summarizing categorical variables as percentages and continuous variables as means±standard deviations (SD). The sensitivity, specificity and predictive values were calculated from a contingency table and the 95% confidence interval (CI) by adjusted Wald method. The reproducibility was assessed using Kappa statistics (k<0.4 poor agreement, 0.4<k<0.6 moderate, 0.6<k<0.8 good, and k>0.8 excellent). Categorical variables were correlated using chi-square statistics, and continuous variables using Student's t-test. p values<0.05 were considered significant. Statistical Package for Social Sciences version 17.0 (SPSS, Chicago, IL, USA) was used.
ResultsA total of 107 of the 234 patients initially screened were finally included; 127 patients were excluded from the analysis, of them 8 had uncertain histological grade, 75 had no or only one follow-up MRI, 67 underwent biopsy or partial excision instead of curative intent resection, 16 showed no (or very small) residual cavity and 5 received intracavitary chemotherapy; 44 patients fulfilled at least two exclusion criteria. Significant artefacts on FLAIR sequences were identified in one patient.
Within the 107 included patients, 90 (84.1%) presented high-grade gliomas and 17 (15.9%) low-grade gliomas. GB represented the most frequent type, with 70 cases (65.4% of all the series). The mean age of the 107 patients was 53 years (range 23–79); 66 (61.7%) were men.
A total of 543 follow-up MRI exams were analyzed until progression, or until the last follow-up in patients without recurrence. The mean number of MRI exams per patient was 5.1 (range 2–20). Mean diameter of the residual resection cavity was 39.1mm (range 16–88).
Inter-rater agreement was excellent (k=0.94, p<0.001). Intra-rater agreement was complete (k=1, p<0.001).
High-grade gliomasThis group included 70 patients with grade IV tumours (glioblastoma) and 20 with grade III (18 anaplastic astrocytoma and 2 oligodendroglioma). Mean age of the 90 patients was 55.6 years (range 23–79); 56 (62.2%) were men. The mean number of MRI exams until progression was 4.8/patient (range 2–15). Mean diameter of the resection cavity was 38.5mm (range 17–73). Tumour progression occurred in 73 (78.5%), with mean progression-free survival of 8.5 months (SD 7.1, 95%CI, 6.8–10.2). Hyperintense fluid in the resection cavity on follow-up FLAIR images occurred in 53 (58.9%) of the 90 patients, after a mean postsurgical period of 4.5 months (SD 2.8, 95%CI, 3.7–5.3).
Of 73 patients with tumour progression, 48 (65.7%) showed hyperintense fluid in the resection cavity. Mean progression-free survival was 6.8 months (SD 4.7, 95%CI, 5.4–8.2) for the 48 patients with hyperintense fluid, and 11.7 months (SD 9.5, 95%CI, 7.8–15.6) for the other 25 patients without hyperintense fluid. In 26 (35.6%) patients, hyperintense fluid and progression were observed in the same study (Fig. 1). In 22 (30.1%) patients, hyperintense fluid preceded the detection of progression (Fig. 2), with a mean of 4.1 months (SD 2.1, 95%CI, 3.2–5). The presence of hyperintense fluid was significantly correlated with poorer progression-free survival (mean 6.8 versus 11.7 months, p=0.004) in high-grade gliomas.
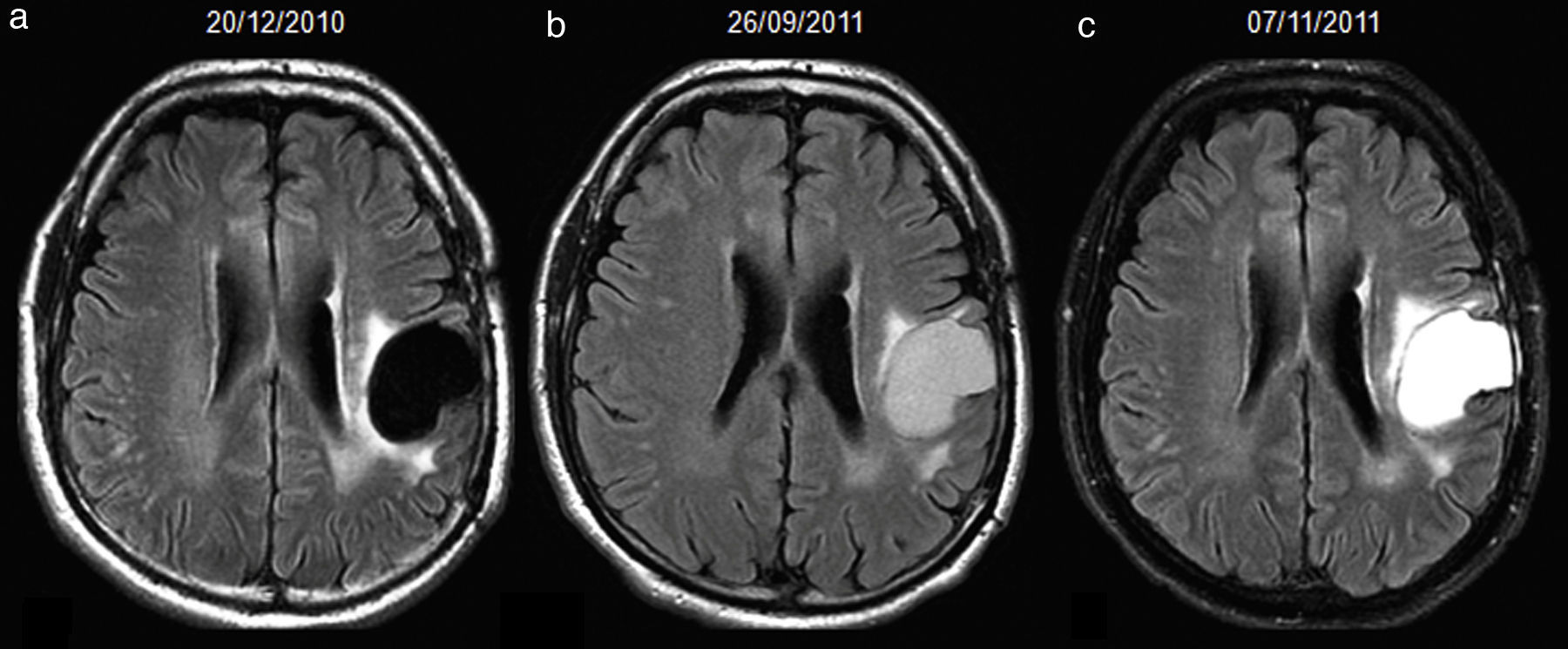
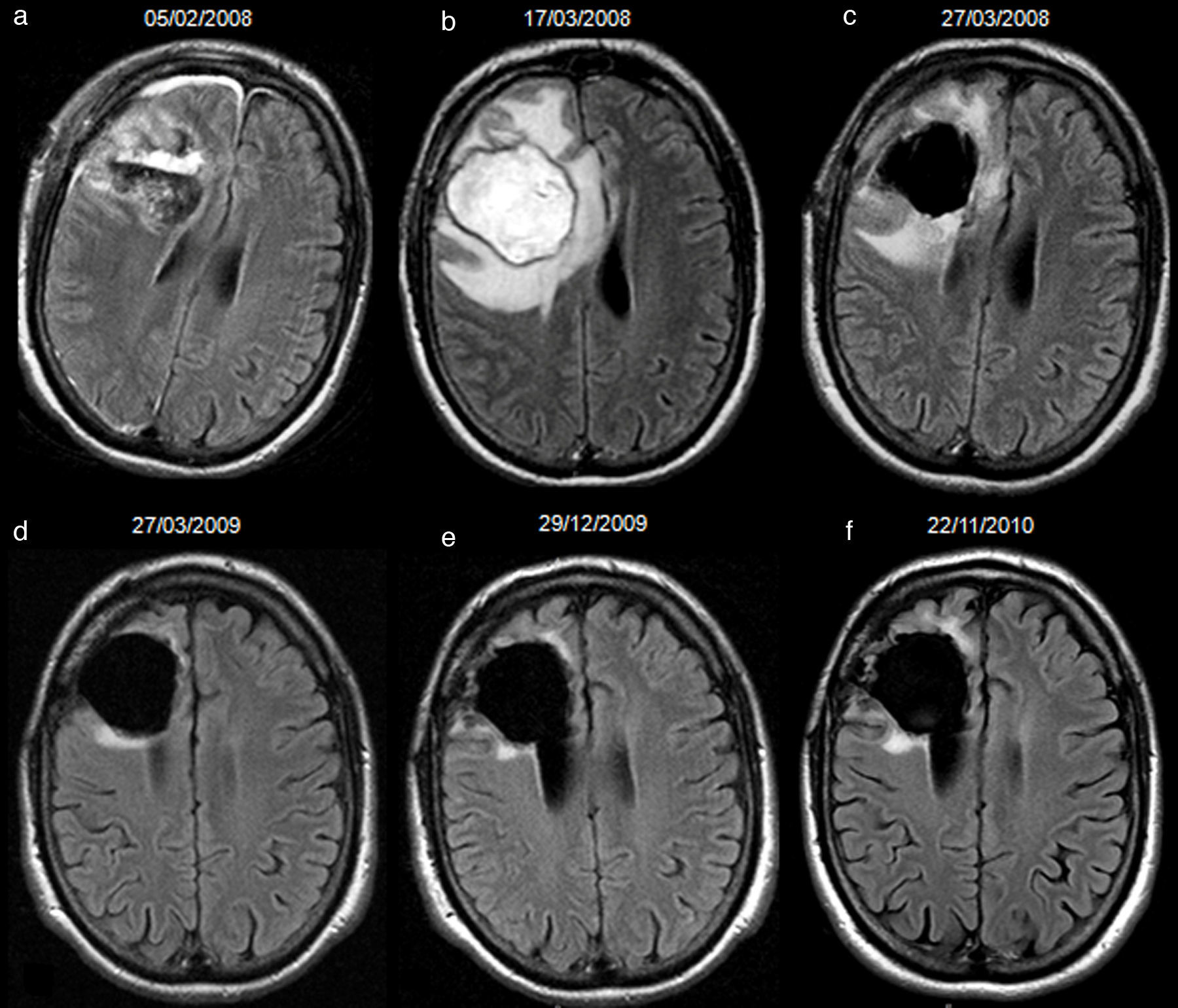
66-year-old man with resected glioblastoma. Hyperintense fluid in the resection cavity in the same follow-up study that demonstrates progression. Follow-up axial FLAIR sequences: (a) at 12 months after surgery, the fluid in the cavity is isointense compared to normal CSF, (b) at 15 months, the fluid becomes hyperintense, and extensive high-signal areas in the posterior part of the cavity are observed and enhance after contrast administration (not shown), consistent with tumour progression, (c) at 18 months, the fluid remains hyperintense and the tumour progresses. CSF-cerebro-spinal fluid.
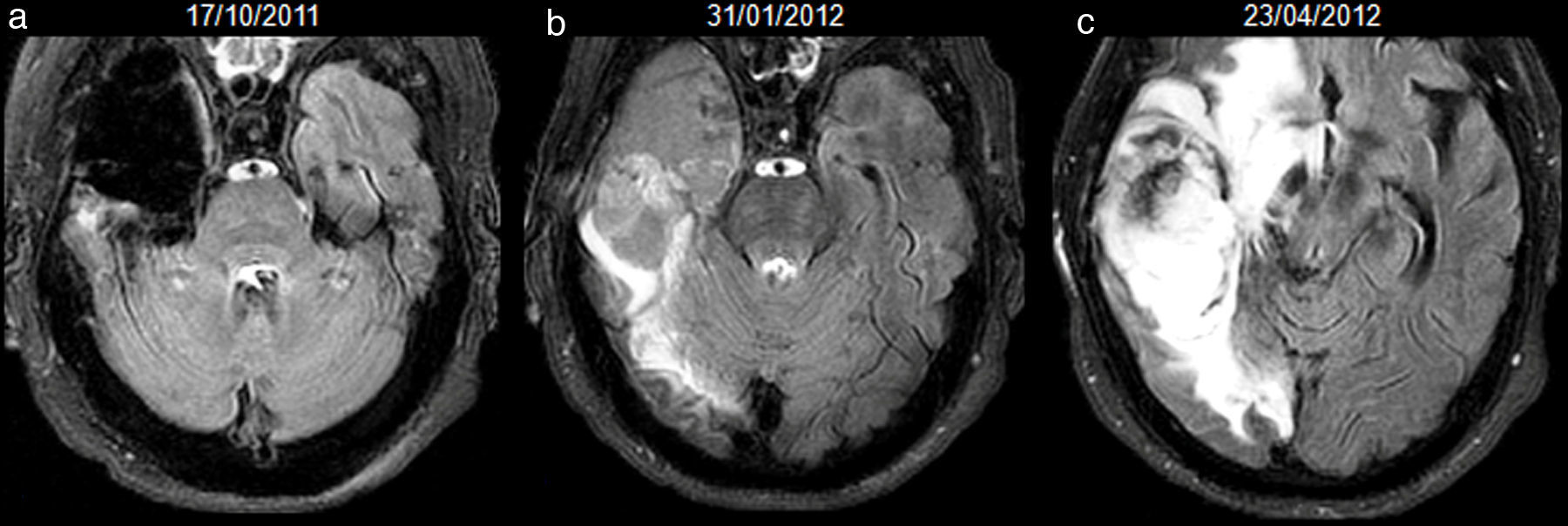
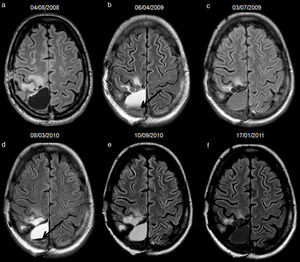
52-year-old man with resected glioblastoma. Hyperintense fluid in the resection cavity before detection of progression. Follow-up axial FLAIR sequences: (a) at 6 months after surgery, the cavity is isointense compared to ventricular CSF, (b) at 15 months, the cavity becomes hyperintense, while no findings reveal tumour progression, (c) at 16.5 months, the cavity remains hyperintense, but high-signal areas in brain parenchyma are slightly more extensive and enhance after contrast administration (not shown), establishing tumour recurrence. CSF-cerebro-spinal fluid.
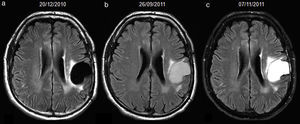
There were 5 cases with hyperintense fluid without progression, considered as false-positives in the analysis. Four were attributed to bleeding (Fig. 3) and 1 to infection. Samples were obtained and cultivated from the cavity of the last patient, and a methicilin-resistant Staphylococcus aureus was found. The postoperative period until hyperintense fluid was identified was between 1 and 3.5 months (mean 2.6) for the 4 patients with intracavitary bleeding and 3 months for the one with infection. All 5 showed resolution of the signal without tumour recurrence in the follow-up studies (mean follow-up 21.5 months, range 11–41). Hyperintense fluid in the cavity predicted progression of the whole group of high-grade gliomas with a sensitivity of 65.7% (95%CI, 54.3–75.6%), a specificity of 70.6% (95%CI, 46.6–87%), positive predictive value of 90.6% (95%CI, 79.3–96.3%) and negative predictive value of 32.4% (95%CI, 19.5–48.6%).
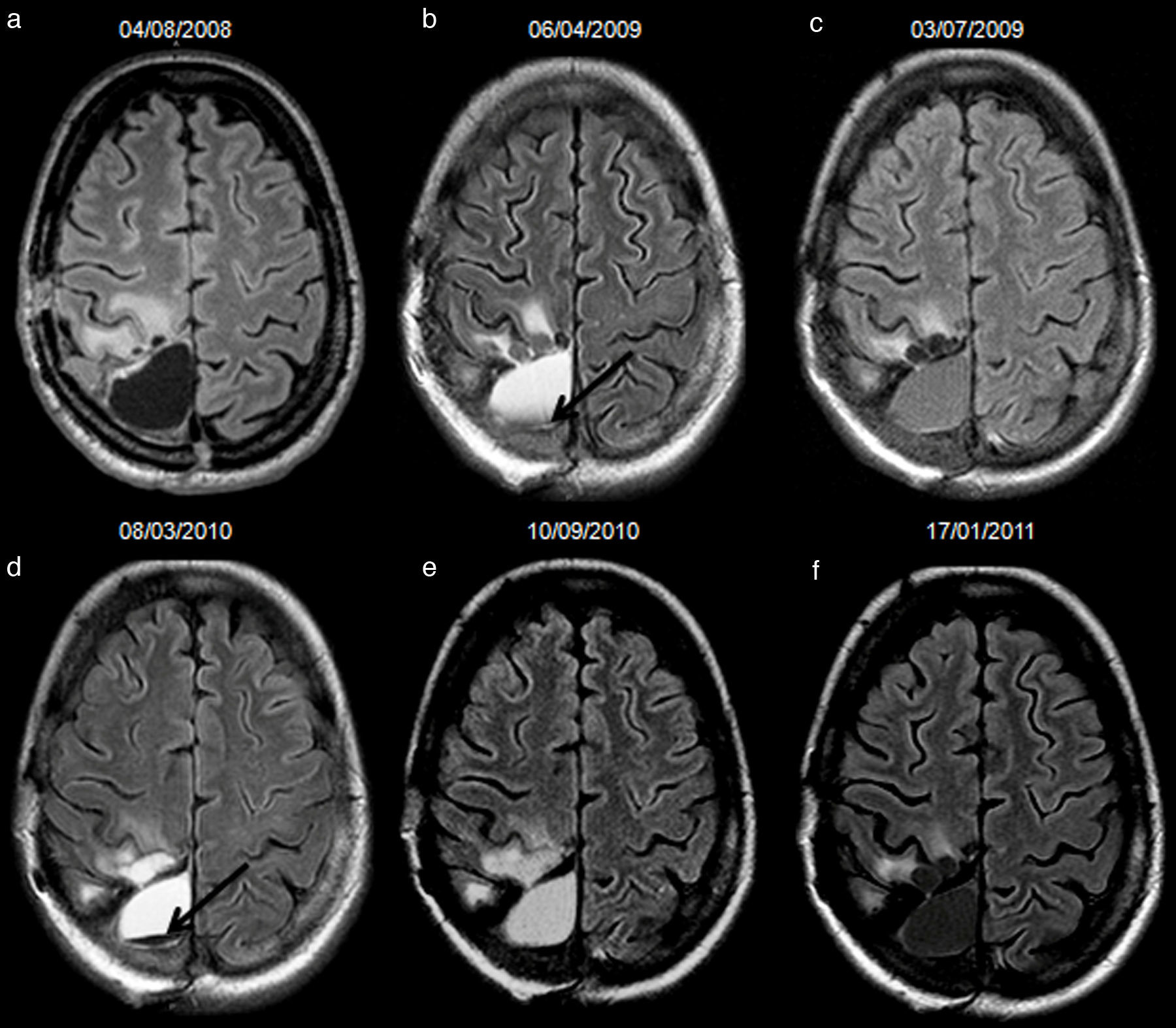
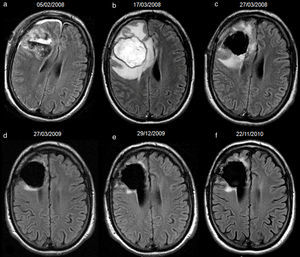
34-year-old woman with resected anaplastic astrocytoma. High-signal attributed to bleeding in the resection cavity, with resolution on subsequent studies, and without tumour progression until the last available study. Follow-up axial FLAIR sequences (a) at 4.5 months after surgery, the cavity is isointense compared to normal CSF, and a hyperintense signal in white matter is present anterior to the cavity, (b) at 12.5 months, the cavity turns hyperintense, with a fluid-fluid level in the posterior aspect of the cavity (arrow); no findings reveal tumour progression on FLAIR or other sequences (not shown), (c) at 15.5 months, the cavity remains slightly hyperintense compared to normal CSF, although it tended to normalize its signal, (d) at 23.5 months, the cavity is hyperintense with a fluid-fluid level in its posterior aspect (arrow) indicating a new episode of bleeding, (e) at 29.5 months, the cavity remains hyperintense compared to normal CSF, (f) at 33.5 months, the signal of the cavity normalizes. CSF-cerebro-spinal fluid.
The analysis was further subdivided in grade III and IV gliomas (Table 1). For grade IV group (GBs), tumour progression occurred in 62 (88.6%) patients, after a progression-free survival of 8 months (SD 6.6, 95%CI, 6.4–9.6). Hyperintense fluid on follow-up FLAIR occurred in 44 (62.8%) patients, after a mean of 4.2 months (SD 2.5, 95%CI, 3.5–4.9). In 19 (27.1%) patients, hyperintense fluid preceded progression, with a mean of 4 months (SD 2.2, 95%CI, 3–5). The presence of hyperintense fluid was correlated with poorer progression-free survival (mean 6.3 versus 11.3 months, p=0.006). There were 3 cases with hyperintense fluid without progression, 2 due to bleeding and 1 to infection. Hyperintense fluid in the cavity predicted progression of GBs with a sensitivity of 66.1% (95%CI, 55–77.2%), a specificity of 62.5% (95%CI, 51.1–73.8%), positive predictive value of 93.2% (95%CI, 87.3–99.1%) and negative predictive value of 19.2% (95%CI, 10–28.4%).
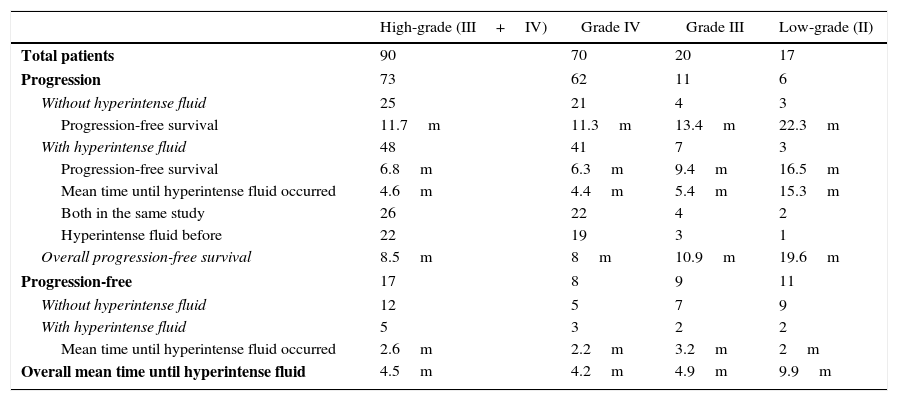
Tumour progression and hyperintense fluid in postsurgical cavity on follow-up FLAIR sequences in resected gliomas. Abbreviations: m-months, FLAIR-fluid-attenuated inversion recovery.
| High-grade (III+IV) | Grade IV | Grade III | Low-grade (II) | |
|---|---|---|---|---|
| Total patients | 90 | 70 | 20 | 17 |
| Progression | 73 | 62 | 11 | 6 |
| Without hyperintense fluid | 25 | 21 | 4 | 3 |
| Progression-free survival | 11.7m | 11.3m | 13.4m | 22.3m |
| With hyperintense fluid | 48 | 41 | 7 | 3 |
| Progression-free survival | 6.8m | 6.3m | 9.4m | 16.5m |
| Mean time until hyperintense fluid occurred | 4.6m | 4.4m | 5.4m | 15.3m |
| Both in the same study | 26 | 22 | 4 | 2 |
| Hyperintense fluid before | 22 | 19 | 3 | 1 |
| Overall progression-free survival | 8.5m | 8m | 10.9m | 19.6m |
| Progression-free | 17 | 8 | 9 | 11 |
| Without hyperintense fluid | 12 | 5 | 7 | 9 |
| With hyperintense fluid | 5 | 3 | 2 | 2 |
| Mean time until hyperintense fluid occurred | 2.6m | 2.2m | 3.2m | 2m |
| Overall mean time until hyperintense fluid | 4.5m | 4.2m | 4.9m | 9.9m |
For grade III group, tumour progression occurred in 11 (55%) patients, after a progression-free survival of 10.9 months (SD 6.8, 95%CI, 6.9–14.9). Hyperintense fluid on FLAIR occurred in 9 (45%) patients, after 4.9 months (SD 2.7, 95%CI, 3.1–6.7). In 3 (15%) patients, hyperintense fluid preceded progression, with 4.7 months (SD 1.9, 95%CI, 2.5–6.8). The presence of hyperintense fluid was correlated with poorer progression-free survival (mean 9.4 versus 13.4 months, p=0.039). There were 2 cases with hyperintense fluid without progression, due to bleeding. Hyperintense fluid in the cavity was associated with the progression of grade III gliomas with a sensitivity and negative predictive value of 63.6% (95%CI, 42.5–84.7%), and a specificity and positive predictive value of 77.8% (95%CI, 59.6–96%).
Low-grade gliomasThis group included 13 patients with astrocytoma, 3 with oligodendroglioma and 1 with oligoastrocytoma. The mean age of the 17 patients was 38.7 years (range 26–53); 10 (58.8%) were men. The mean number of MRI exams until progression was 6.4/patient (range 2–10). Mean diameter of the resection cavity was 42.3mm (range 16–88). Tumour recurrence occurred in 6 (35.3%), with mean progression-free survival of 19.6 months (SD 16.2, 95%CI, 2.6–36.6). Hyperintense fluid in the resection cavity was identified in 5 (29.4%) subjects, and the mean postsurgical period until the fluid turned hyperintense was 9.9 months (SD 16.3, 95%CI, 0–30). In 1 of 6 patients with recurrence, hyperintense fluid occurred 6 months before (Table 1).
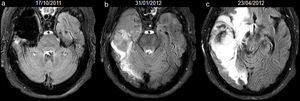
False-positives were present in 2 cases: 1 due to bleeding in the cavity identified at 3 months after surgery and 1 to infection 1 month after surgery (Fig. 4). In the last patient, the cavity was drained and pus-like material was obtained and cultivated; coagulase-negative staphylococci and corynebacterium species were found in three different samples. Both false-positives showed resolution of the signal without tumour recurrence in the follow-up (32 months and 34 months, respectively). Hyperintense fluid in the cavity was associated with the recurrence of low-grade gliomas with a sensitivity of 50% (95%CI, 18.7–81.2%), a specificity of 81.8% (95%CI, 51.1–96%), positive predictive value of 60% (95%CI, 22.9–88.4%) and negative predictive value of 75% (95%CI, 46.1–91.7%).
40-year-old man with resected oligodendroglioma. High-signal due to abcessified fluid in the resection cavity, with resolution on subsequent studies, and without tumour recurrence until the last study included in the analysis. Follow-up axial FLAIR sequences (a) at 4 days after surgery, the signal of the cavity is heterogeneous due to debris and haemorrhage, (b) at 1.5 months, the cavity is hyperintense compared to ventricular CSF; the association with prominent oedema, ring-enhancement, restricted diffusion and low rCBV (not shown) indicates an abscess in the cavity; no findings reveal tumour recurrence on FLAIR or other sequences (not shown), (c) after draining the abscess and 10 days later, the signal of the cavity turns isointense compared to ventricular CSF, (d–f) subsequent studies at 14, 23 and 34 months, the cavity remains isointense. CSF-cerebro-spinal fluid, rCBV-relative cerebral blood volume.
Overall, hyperintense fluid occurred more commonly (58.9% versus 29.4%, p=0.025) and earlier (mean postoperative period of 4.5 months versus 9.9 months, p<0.001) in high-grade than in low-grade gliomas.
DiscussionThe present study analyzes the signal of fluid in the postsurgical cavity on follow-up FLAIR sequences in a large cohort of resected gliomas. High-grade gliomas were the dominant group in our series, especially the GBs. Hyperintense fluid in the resection cavity occurred in more than half of them, and in almost two-thirds of those that eventually progressed. Importantly, in 30% of the high-grade gliomas with progression, hyperintense fluid was observed with a mean of 4 months before. This last subgroup could be the one with the highest utility of this finding. Low-grade gliomas showed hyperintense fluid in the resection cavity in almost one-third of them. Overall, hyperintense fluid occurred more frequently and earlier in high-grade gliomas than in low-grade ones, which is something expected for a sign related to disease progression, given that high-grade gliomas progress before and more frequently than low-grade gliomas. Within the group of high-grade gliomas, the occurrence of hyperintense fluid was correlated with poorer progression-free survival. This could be crucial for closer monitoring, therapeutic decisions, and outcome. Furthermore, assessment of signal in the cavity was very quick and reproducible.
Higher frequency and earlier presentation of hyperintense fluid in high-grade gliomas, together with a lower progression-free survival when hyperintense fluid occurred, indicates more aggressive mechanisms and poorer outcome in gliomas that associate hyperintense fluid in the resection cavity. An explanation for the high-signal of fluid could be an adjacent neoangiogenesis with a leak of different products into the cavity through the immature walls of the neo-vessels, which could lead to a high level of proteins or microhaemorrhagic products in the fluid. Another explanation, previously postulated, could be the slight growth of the tumour with impairment of the exchanges between CSF from the resection cavity and surrounding CSF spaces.9
Overall, our results confirm the main finding of the previous studies,9,10 that high-signal in the resection cavity on FLAIR images is associated with the progression of gliomas and can predict the progression in some cases, and this can add a significant tool for clinical practice. However, sensitivity and specificity in the present study were moderate to good, and a very high specificity as in the previous studies was not reached. Nevertheless, even if the specificity in our study was limited, the false positives were easily recognized, as shown by the inter- and intra-observer agreement. The specificity was higher for low-grade gliomas, and probably including a higher sample of low-grade gliomas in our study would have led to similar results to those formerly reported.9 We found a very good positive predictive value (90.6%) of this sign for high-grade gliomas, which was even higher (93.2%) for the GBs group. Another difference is that in both previous studies tumour progression was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, which are based only on the unidimensional measurement of target lesion and are not specific for brain tumours, while we used RANO criteria in an attempt to provide a more precise and operational assessment of gliomas progression. This and the high proportion of low-grade gliomas can explain the higher interval with which hyperintense fluid preceded progression (5 months) in the first study.9 On the other hand, in the second study,10 consisting of high-grade gliomas, this interval was lower (around 2 months).
In our study, false-positives of hyperintense fluid in the resection cavity were identified. They were due to infection and bleeding, and all of them showed a trend towards occurring early in the postoperative period. Infection of the cavity was demonstrated in two patients. The occurrence in the first period after surgery, and the types of pathogens cultivated in both patients are indicative of perioperative contamination. Bleeding in the cavity occurred in 5 patients, of which 4 were high-grade gliomas. Follow-up MRIs showed resolution of the signal, without tumour recurrence. The association with haemorrhage is another difference between high-grade and low-grade gliomas; in GB bleedings occur in more than half of cases, while in low-grade gliomas are uncommon.11,12 Therefore, infection and haemorrhage have to be ruled out first in order to consider progression.
The present study has several potential limitations. Firstly, the retrospective nature, and the limited number of low-grade gliomas. Secondly, the acquisition of MRI studies. About half of the patients were scanned at 3T, which is known to produce more artefacts on FLAIR sequences.13 Significant artefacts that could have hampered correct assessment were identified in only one patient, excluded from the analysis. In the rest of the studies, this was not a limitation, as showed by the inter-rater and intra-rater reliability. Also, the signal in the cavity was assessed by comparison to ventricular CSF within the same study, and we did not compare FLAIR images from different studies or different scans, so this should not affect the analysis. Thirdly, we did not include data about possible dysfunctions of homeostasis, immunosuppression or other disorders, therefore their possible influence cannot be ruled out. Lastly, a potential confounding factor is that statistically, almost all gliomas progress, especially the high-grade ones. This can be translated into a high probability of coincidence between progression and hyperintense fluid due to other causes such as haemorrhage, and this probability could be higher when the total follow-up period is longer. Despite these potential limitations, the present study can be representative in current clinical settings.
In conclusion, our study confirms that high-signal fluid in the resection cavity on follow-up FLAIR sequences is associated with progression and can predict progression in gliomas. Hyperintense fluid in the resection cavity occurred more frequently and earlier in high-grade gliomas, and predicted poorer progression-free survival. False-positives due to bleedings and infection can be observed, and are usually conspicuous.
Conflict of interestWe declare that we have no conflict of interest.