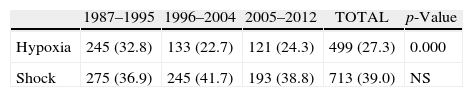To describe the demographic and clinical profiles of a cohort of environmentally representative severe traumatic brain injury (TBI) cases collected for the past 25 years and to analyse the changes that occurred by dividing the analysis period into 3 equal time periods.
Material and methodsThis was an observational cohort study of consecutive adult patients (>14 years of age) with severe closed TBI (Glasgow Coma Scale score [GCS]≤8) who were admitted during the first 48h after injury to the 12 de Octubre hospital from 1987 to 2012. The most relevant epidemiological and clinical variables reported in the literature were defined and compared in 3 equal time periods (1987–1995, 1996–2004 and 2005–2014).
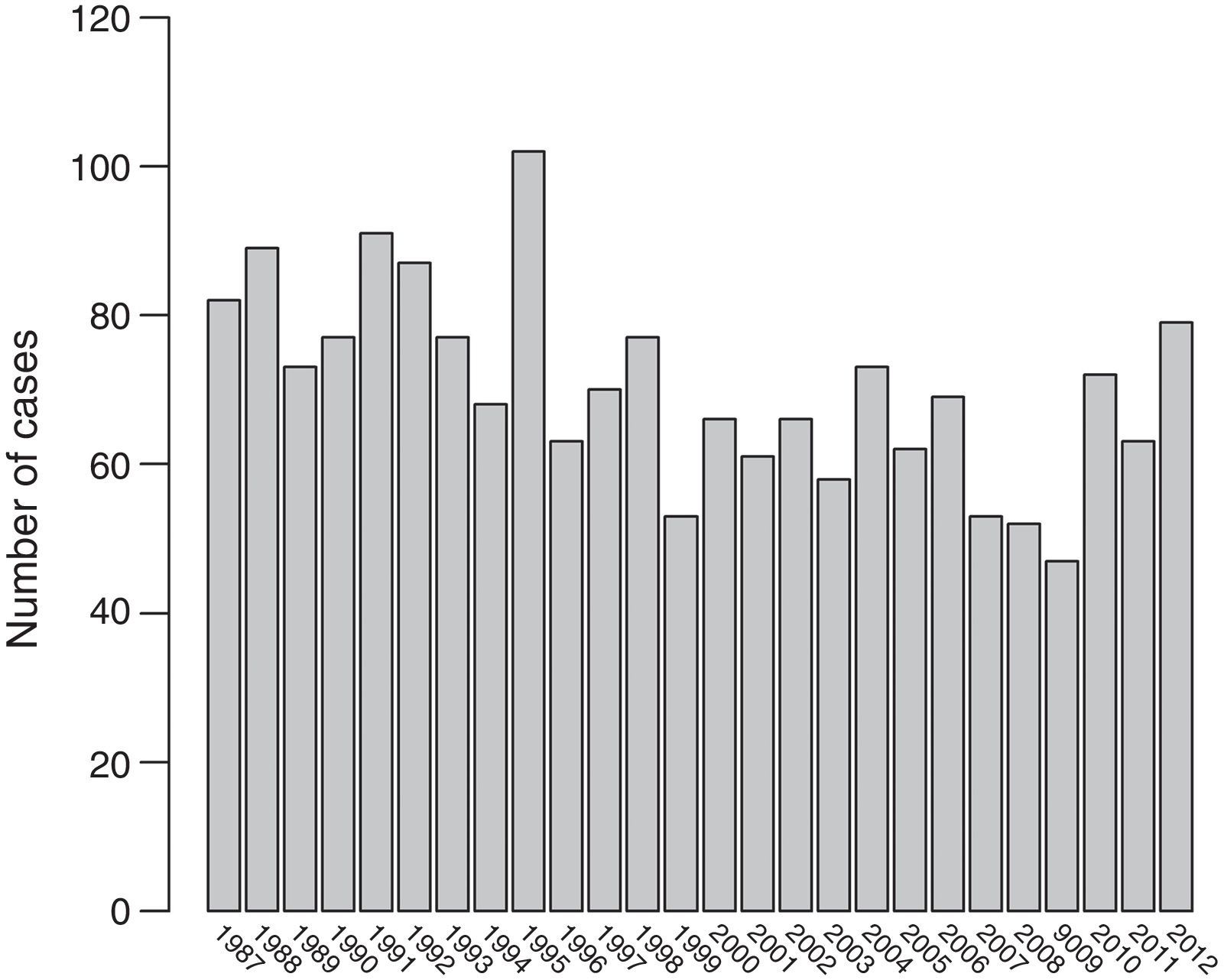
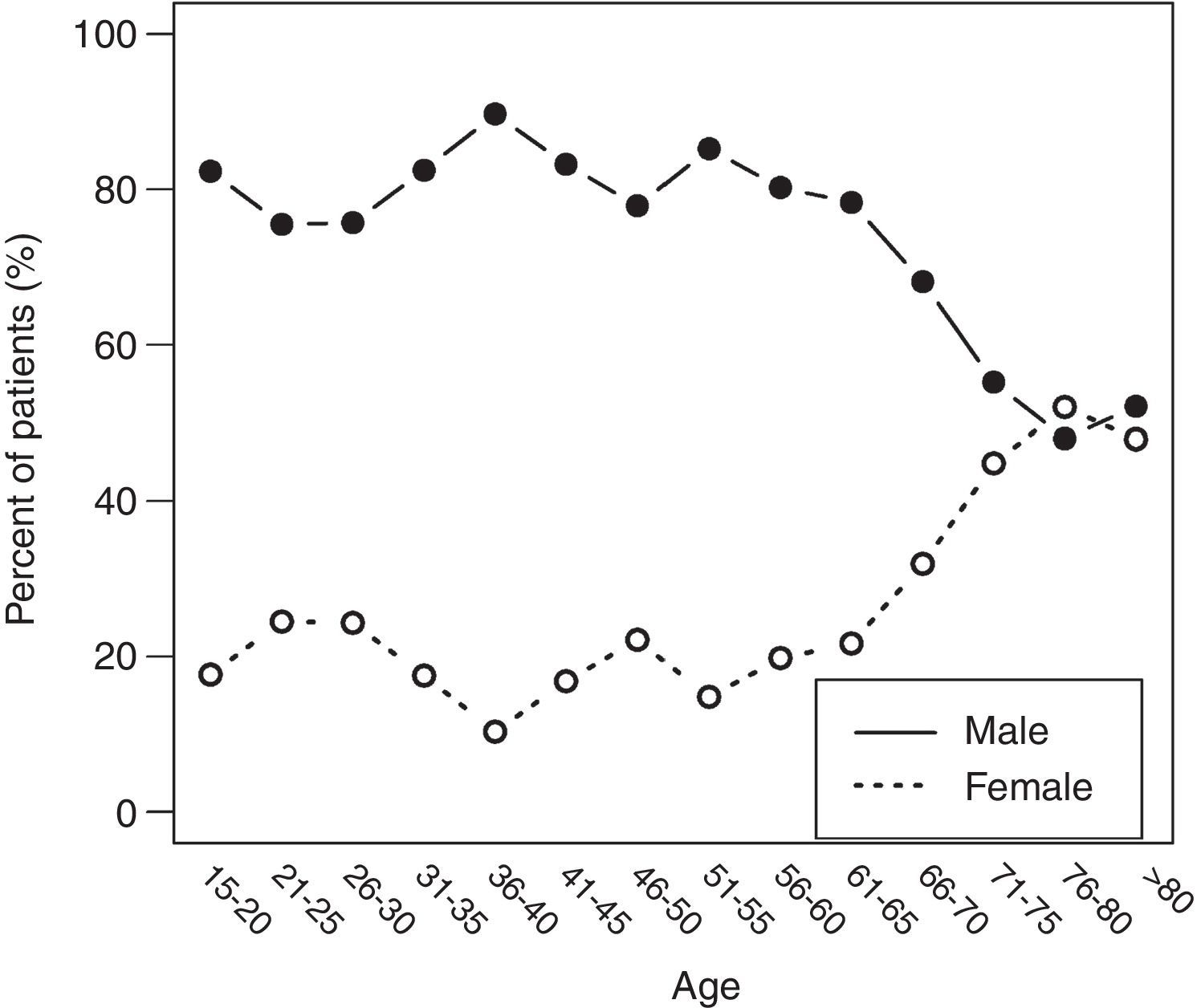
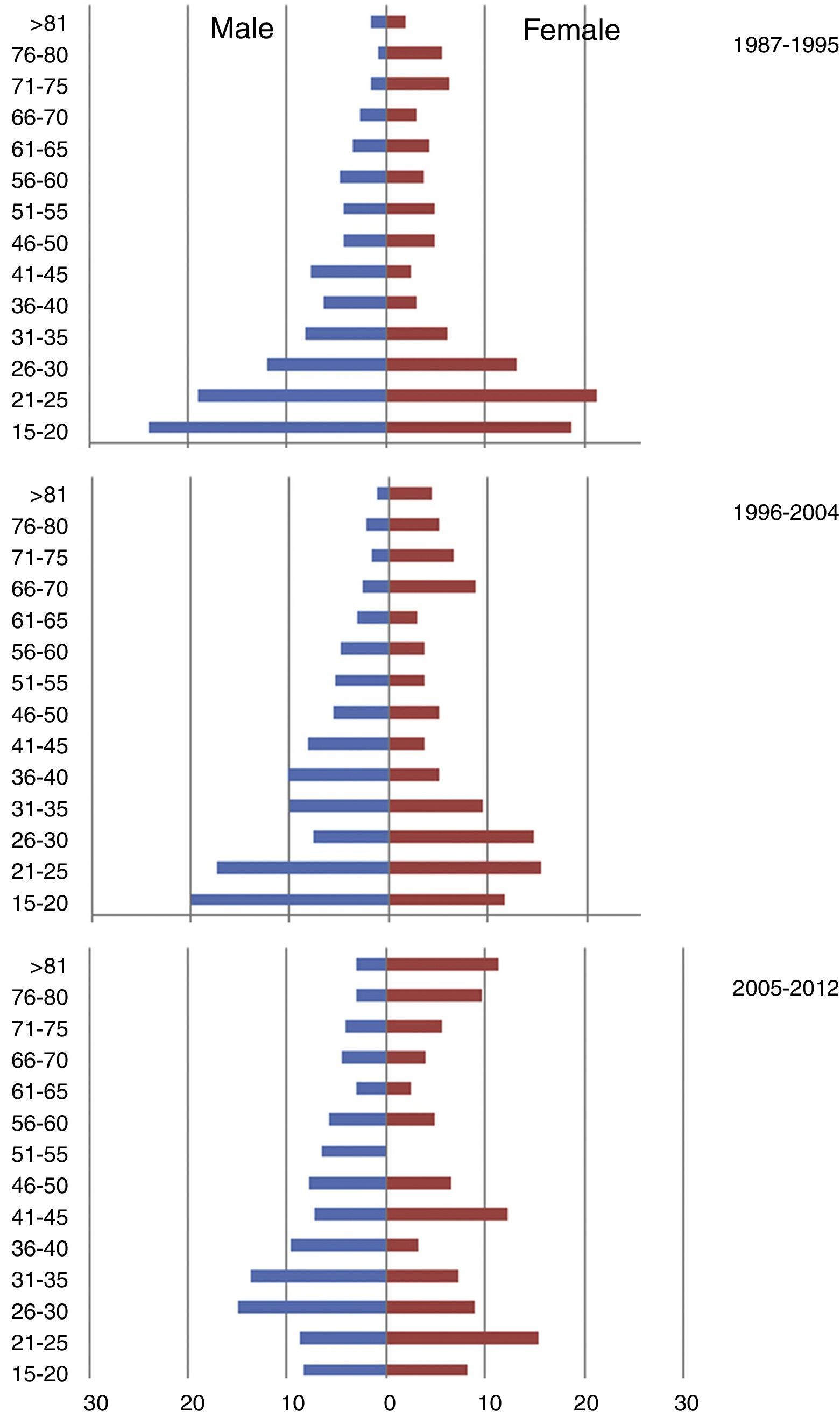
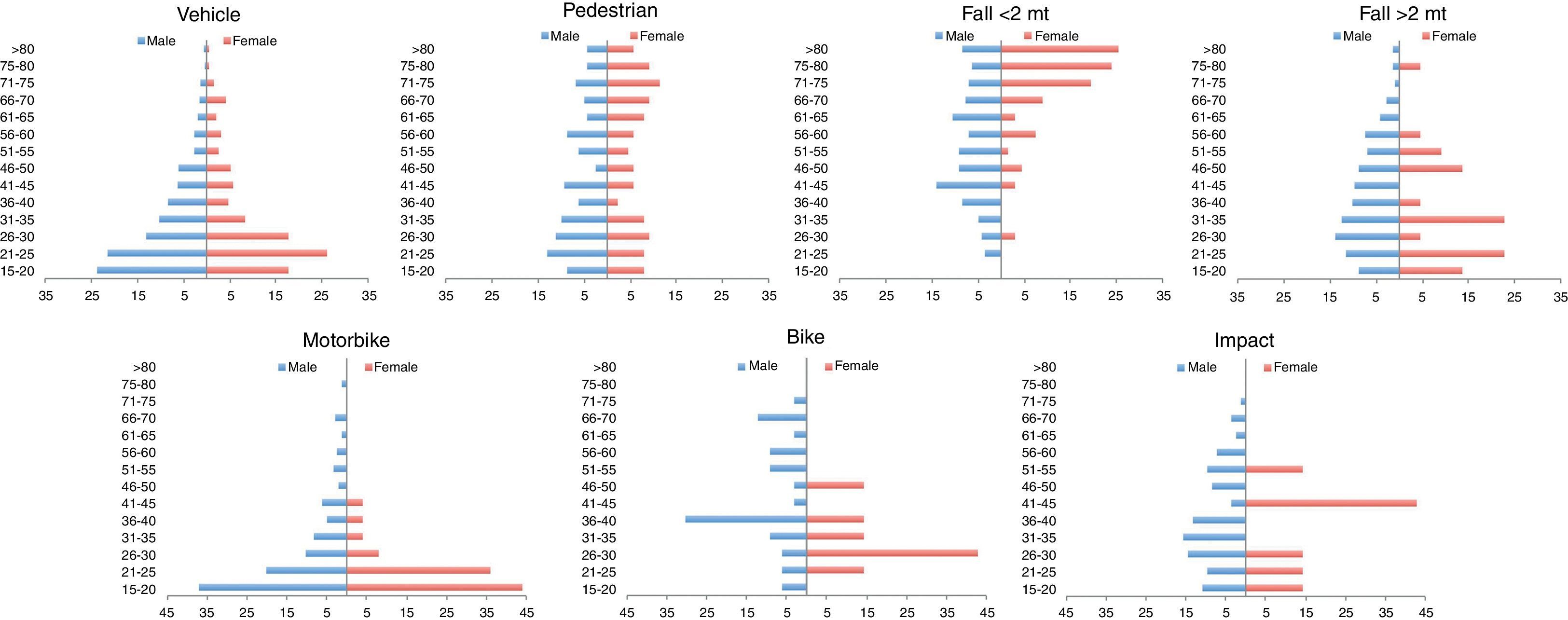
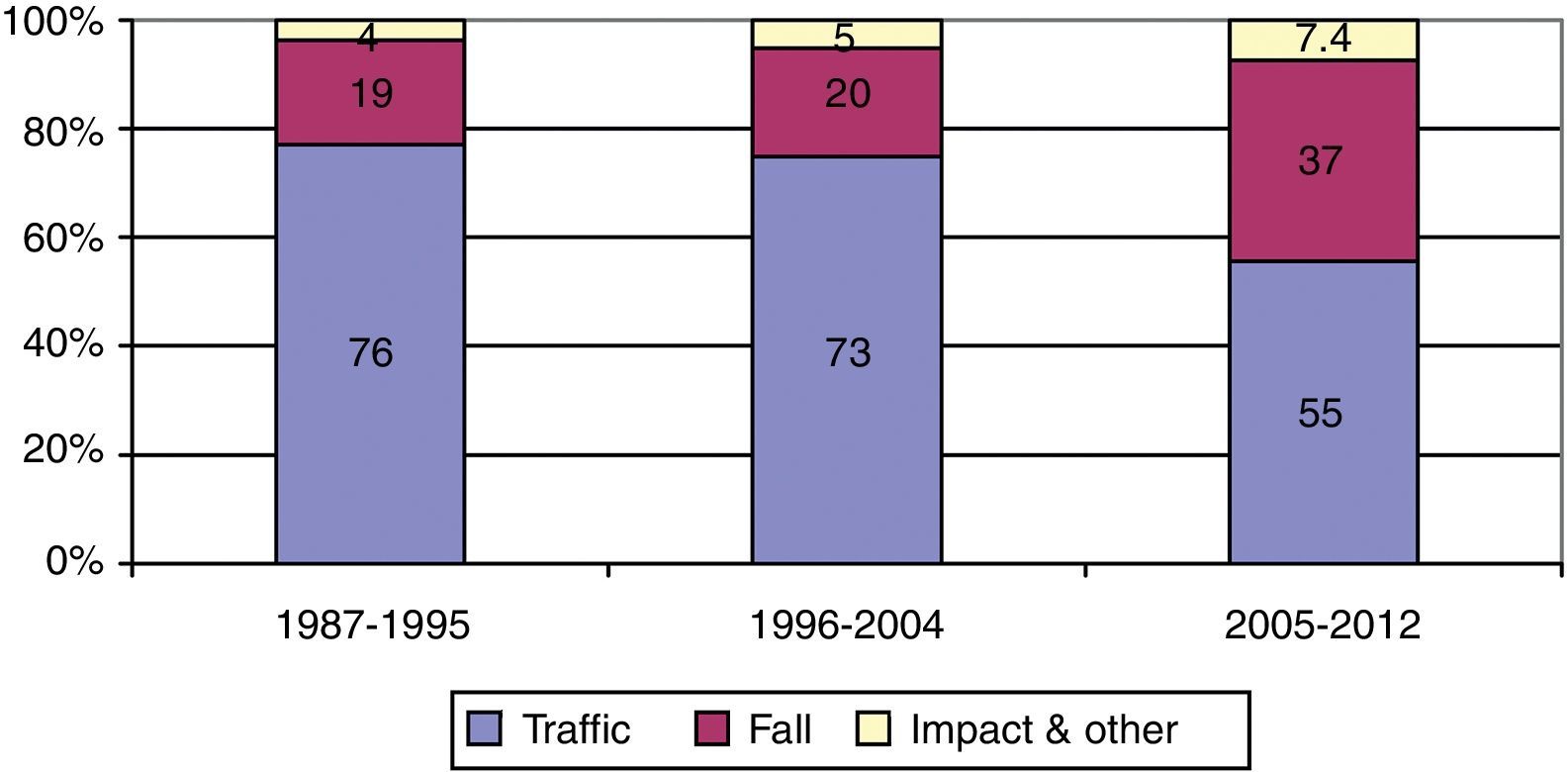
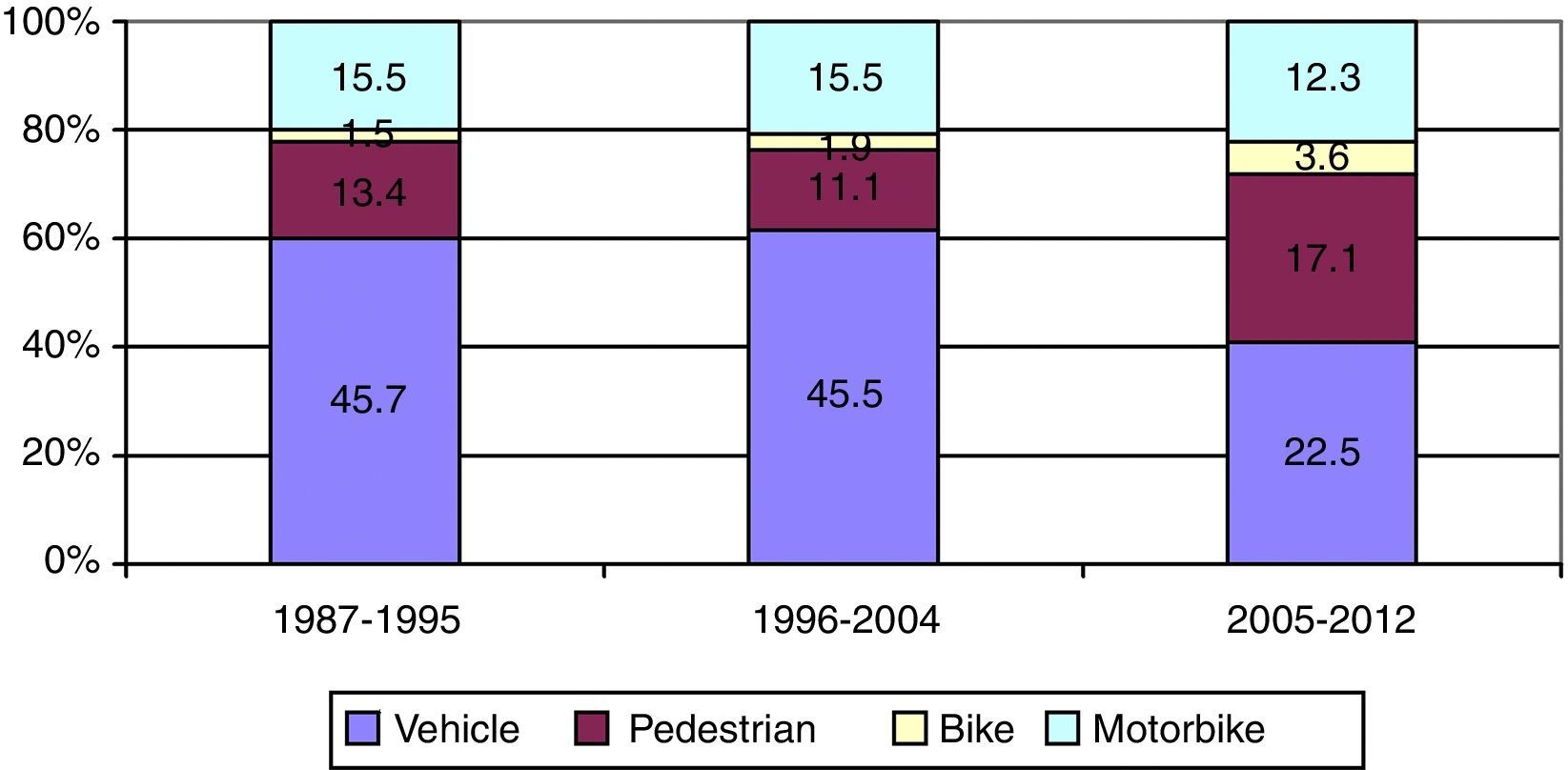
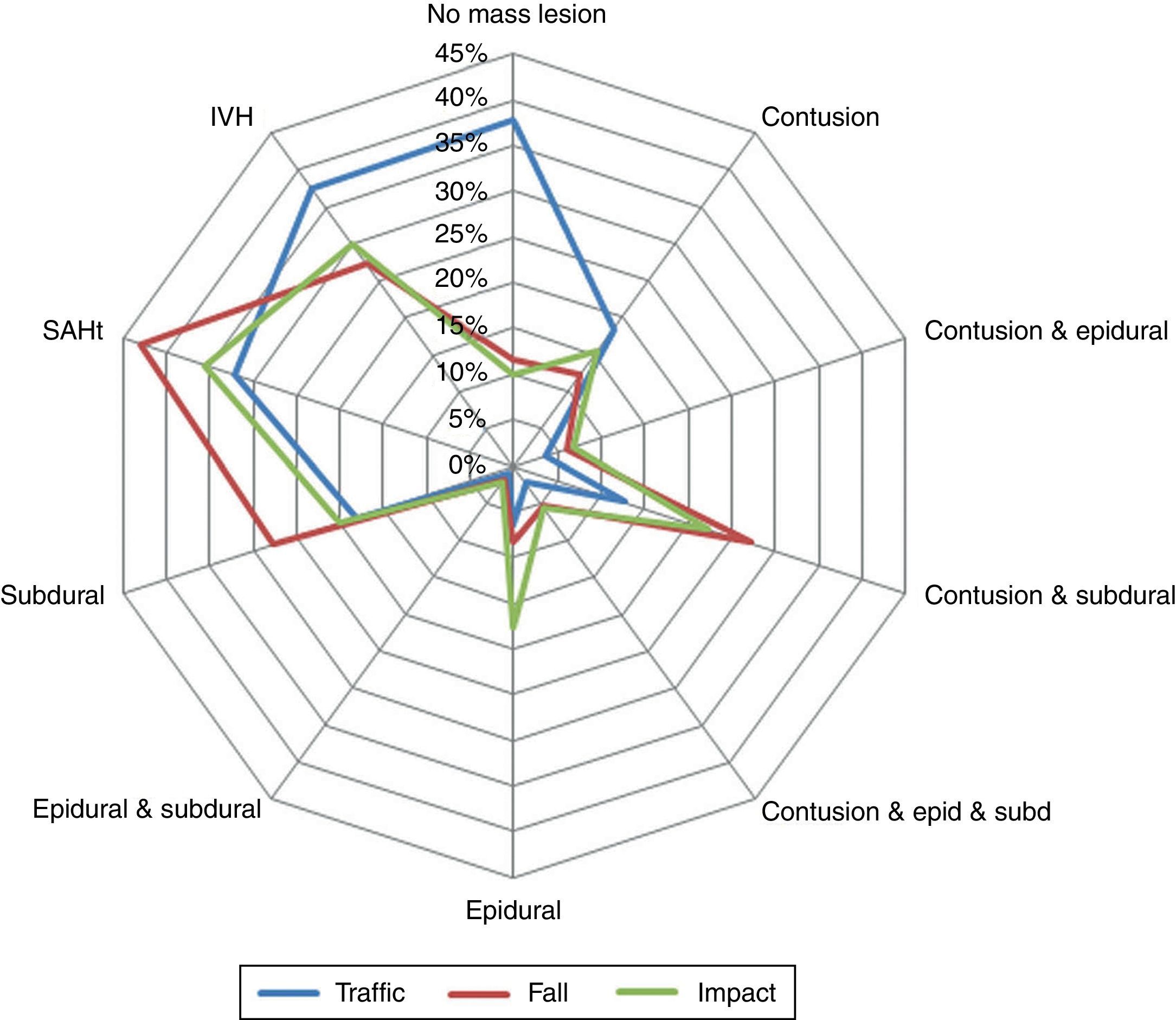
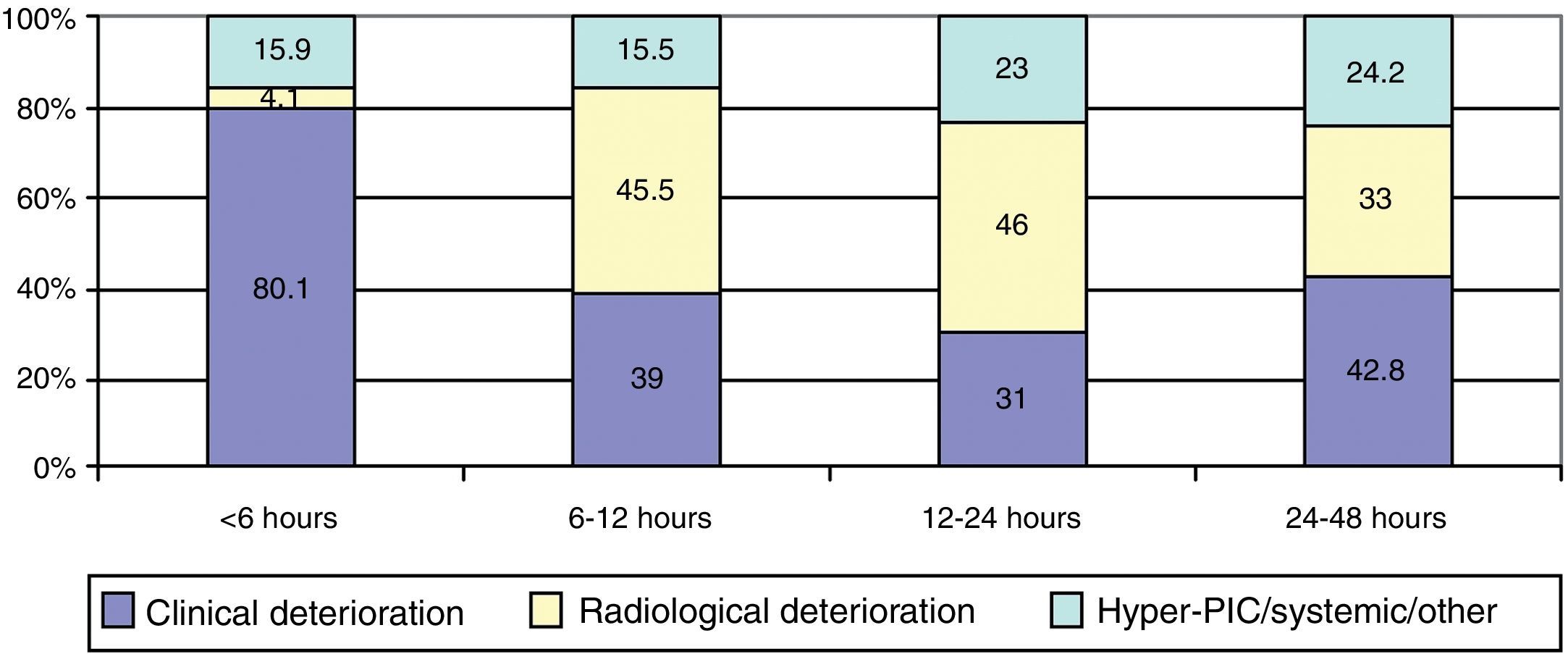
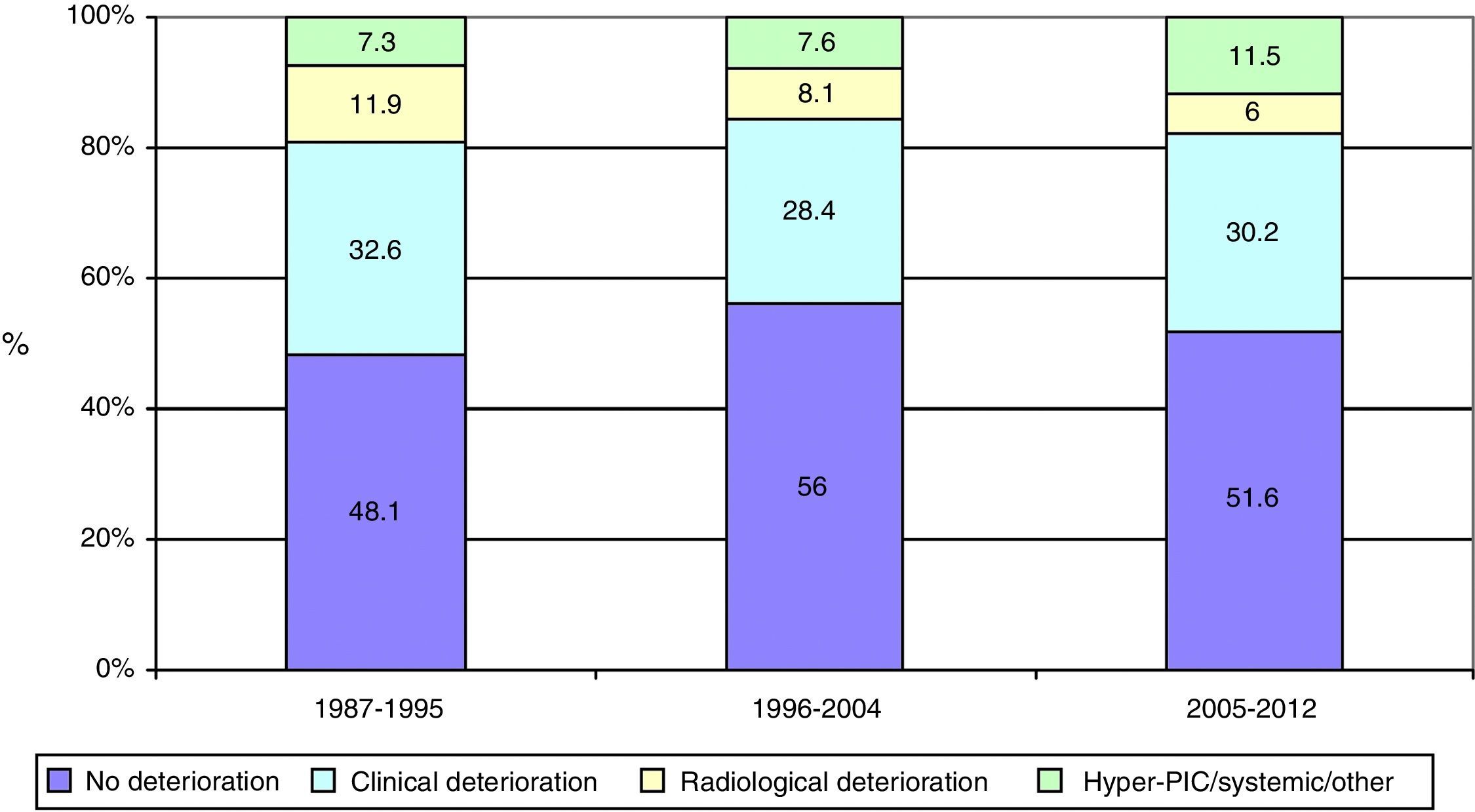
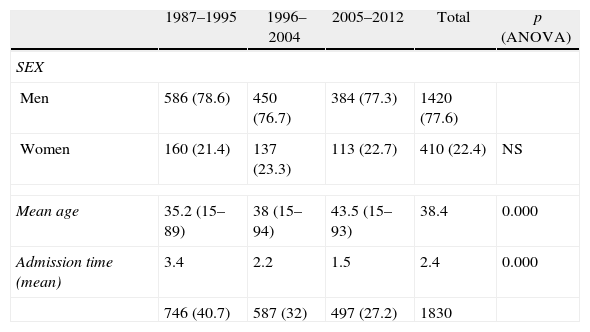
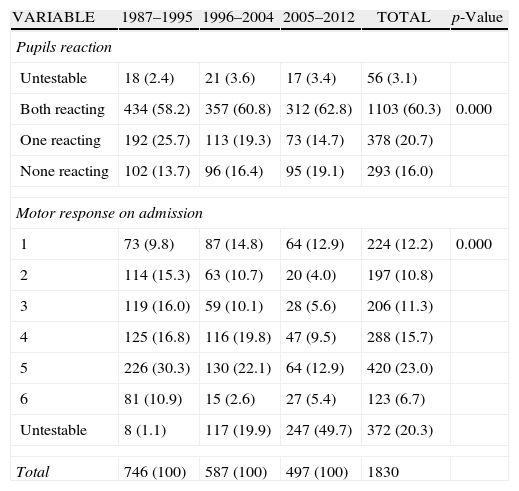
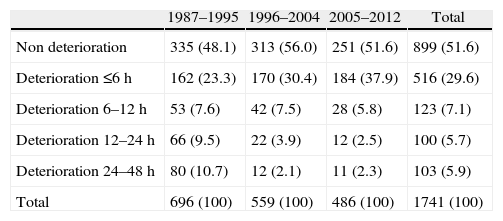
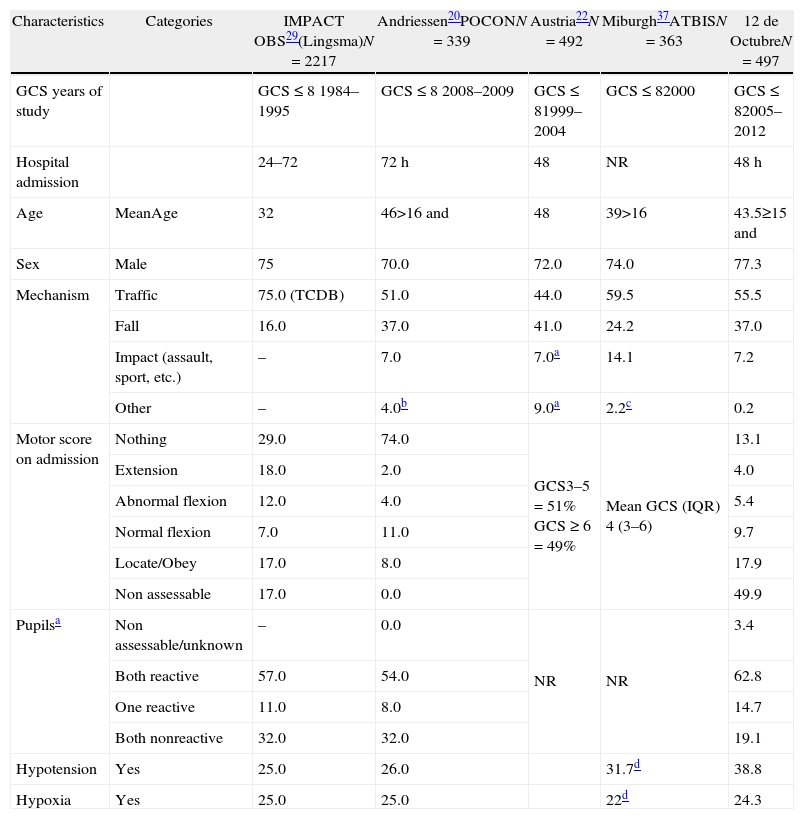
ResultsThere was a 13% reduction in the frequency of severe TBI from the first to the last time period. An increase in the mean age from 35 to 43 years was observed, whereas the frequency of severe TBI according to sex remained approximately the same during the last decades of life. A distinct change was observed in the injury mechanism; traffic accidents decreased from 76% to 55%, particularly those involving 4-wheeled vehicles. However, falls increased significantly, especially in older women, and contusion and subdural haematoma were the most frequent structural injuries. Motor scores could not be reliably assessed for the last time period because of early intubation and sedative drug use.
ConclusionsTBI epidemiology in Western countries has changed. This trend was also observed in our environment as an increase in mean age, which reflected the increase in falls among elderly patients.
Describir el perfil demográfico y clínico de una cohorte de TCE grave recogida en los últimos 25 años, representativa de nuestro medio y analizar los cambios que han sucedido a lo largo de estos años, dividiéndolos en tres periodos de tiempo iguales.
Material y métodosEstudio observacional de cohorte de TCE grave cerrado (puntuación en la escala de Glasgow GCS≤8) adultos (>14 años) ingresados consecutivamente en las primeras 48 horas del traumatismo, en Hospital 12 de Octubre entre 1987 y 2012. Se definen las variables epidemiológicas y clínicas que han demostrado ser las mas relevantes en la literatura y se comparan en 3 periodos de tiempo equivalentes, 1987-1995, 1996-2004 y 2005-2014.
ResultadosExiste una reducción de la frecuencia de TCE grave de un 13% entre el primer y último periodo de tiempo. Se aprecia un aumento de la edad media de 35 a 43 años, la frecuencia de TCE grave por sexo se iguala en las últimas décadas de la vida. Se aprecia un cambio en el mecanismo del trauma, el accidente de tráfico ha disminuido de un 76% a un 55%, sobre todo se ha reducido el accidente en vehículo-4 ruedas. Sin embargo, han aumentado notablemente las caídas, especialmente en mujeres de mayor edad, siendo la lesión estructural mas frecuente la contusión y el hematoma subdural. En el último periodo de tiempo no se pudo determinar con fiabilidad la puntuación motora, debido a la intubación precoz y el uso de drogas sedantes.
ConclusionesLa epidemiología del TCE ha cambiado en los países occidentales, esta tendencia también se observa en nuestro medio, con un aumento de la edad media que refleja el aumento de caídas en la población de pacientes de edad avanzada.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.