Chronic pain is one of the most prevalent pathologies in the world. Treatment with neurostimulators is carried out in the most extreme cases and requires a large investment of resources. In these times of the COVID19 pandemic, we present a comprehensive solution for monitoring this kind of patient, this solution includes the development of a mobile application and a support center for remote monitoring (SCRM).
Material and methodologyThe project was developed according to the scientific evidence in the following phases: (1) Approval in a multidisciplinary clinical committee of implants for chronic pain, (2) Setting up a group of experts, (3) Protocol adaptation for the follow-up of patients with chronic pain to the Smartphone environment, (4) Technology platform adaptation to the clinical protocol (technological environment and workflow between the hospital and the SCRM), and (5) Quality evaluation by survey (quantitative and qualitative) of a small series of patients.
ResultsThe application was evaluated by asking for user opinions about design and usefulness with the first implanted patients. Some minor adjustments were made concerning downloadable material and screen color and text.
ConclusionsDeveloping a comprehensive solution should be based on scientific principles and in accordance with established protocols. A support center ensures greater adherence for follow-up and better patient care.
El dolor crónico es una de las patologías más prevalentes en el mundo. El tratamiento con neuroestimuladores se realiza en los casos más extremos tras una cuidadosa selección, y demanda una gran inversión de recursos en su seguimiento. En estos momentos de pandemia por la COVID19, presentamos una solución integrada para el seguimiento de estos de pacientes, que incluye el desarrollo de una aplicación para dispositivos móviles y un centro de soporte para seguimiento remoto (CSSR).
Material y metodologíaEl proyecto se ha desarrollado basándose en evidencia científica en las siguientes fases: 1) Aprobación de la idea en sesión clínica multidisciplinar de implantes para dolor crónico, 2) Formación de un grupo de expertos, 3) Adaptación del protocolo para el seguimiento de los pacientes con dolor crónico a las características del entorno de un Smartphone, 4) Adaptación de la plataforma tecnológica al protocolo clínico (entorno tecnológico y flujo de trabajo entre el hospital y el CSSR) y 5) Evaluación de la calidad mediante encuesta (cuantitativa y cualitativa) con una pequeña muestra de pacientes.
ResultadosLa aplicación de paciente se evaluó solicitando opiniones de los usuarios sobre el diseño y la utilidad de la misma entre los primeros pacientes implantados que la usaron. Se realizaron algunos ajustes menores en relación con el material para descargar y sobre el texto y el color de la pantalla.
ConclusionesEl proceso de creación de una solución integrada debe estar basado en principios científicos y acorde con los protocolos establecidos. Un Centro de Soporte permite asegurar una mayor adherencia al seguimiento y una mejor atención a los pacientes.
The management of chronic pain is still currently one of the most significant challenges that a doctor can face in their regular clinical practice. According to data from the Ministry of Health in Spain, it is estimated that low-back pain has a prevalence of 19%, followed by osteoarthritis at 18% and neck pain at 16%1. It affects more than 500 million people worldwide and is the leading cause of years lived with disability2. The current COVID-19 pandemic has only aggravated the situation for these patients. It is well known that the different alert levels, with lockdowns and the existing restrictions on mobility, condition a worsening of the clinical situation of those who suffer from chronic pain3.
For patients with persistent lumbar/radicular pain, after conservative treatments have been exhausted, posterior or medullary epidural spinal cord stimulation (SCS) treatment has been employed, with improvement rates considered to be 50/50 (50% improvement in 50% of patients)4. In addition, these patients consume a large part of the financial resources of pain units and/or neurosurgery departments, and in the COVID-19 pandemic environment, it seems logical to make an effort to carry out the best possible follow-up to make the treatment a more profitable investment.
In recent years, multiple digital tools have appeared to fill this gap and promote patient follow-up, from websites (eHealth) to mobile applications (mHealth), and they are increasingly being used as a tool by healthcare professionals to provide a better service to their patients5. These eHealth or mHealth instruments have not yet been fully developed, since in many cases scientific rigour is lacking6. However, in the next few years they will be integrated into routine clinical practice and must be taken into account as a powerful tool for improving health care.
The objective of this study is to determine the feasibility of the implementation of an integrated remote care programme for patients suffering from chronic pain — specifically those with implantable neurostimulators — without causing an exponential increase in the workload of the healthcare professionals involved. This programme involves a technological platform with a mobile application for patients, a website for professionals and a remote patient monitoring support centre (RPMSC) made up of a team of non-hospital professionals in charge of implementing a set of professional support services.
Material and methodsTo evaluate the project from a scientific perspective, it was decided to follow the John Hopkins University tool for the development of clinical applications7. This was adapted to our setting and to the project (Table 1).
Project phases.
| John Hopkins tool steps | Project phases |
|---|---|
| 1. Define the problem and the digital tool | 1. Approval of the idea about implants for chronic pain in a clinical session |
| 2. Creation of a multidisciplinary group to analyse the problem | 2. Constitution of a group of experts |
| 3. Seeking an opportunity to accelerate the project | 3. Adaptation of the clinical and educational protocol |
| 4. Involving patients | 4. Adaptation of the technological platform to the clinical protocol |
| 5. Consulting different partners | 5. Quality assessment |
| 6. Conducting a clinical validation |
Source: Adaptation to our setting of the tool for the development of clinical applications designed by John Hopkins University, equating each of its phases to those of our project.
At our centre, multidisciplinary sessions on chronic pain are held with professionals from different clinical management units (anaesthetists, emergency physicians, neurophysiologists, neurologists, rehabilitation physicians and neurosurgeons) with the aim of evaluating each proposed case individually to decide on the best treatment available for each occasion, making decisions on implantable neurostimulators, infusion pumps, percutaneous techniques, experimental therapies, etc.
While taking into account the types of patient and such complex conditions, without forgetting about the coronavirus (COVID-19) pandemic that we have been immersed in since the beginning of 2020, it was decided to look for an alternative to improve the healthcare process. It was initially decided to apply the project to the subgroup of patients who received a spinal neurostimulator. Based on the results obtained, it was subsequently decided to continue with patients with morphine and/or baclofen pump implants, as well as a final phase for follow-up after performing percutaneous tests.
Constitution of the expert groupAfter approval to start the project from the multidisciplinary committee, a group of experts was chosen to begin defining the main guidelines for the project. The group of experts was made up of five professionals from the hospital centre from various clinical management units, as well as several project management professionals experts in patient-oriented digital solutions. The project was developed based on the experience these managers had regarding the design and implementation of integrated healthcare solutions for other patient profiles (insulin infusion pumps, cardiac devices, monitoring during home isolation due to coronavirus, etc.).
Adaptation of the clinical and educational protocolThe monitoring protocol used by the centre since 2018 was taken up and modified by the group of experts in order to expand and adapt it to the new digital platform environment. This protocol was adapted to the characteristics of neurostimulator patients and had previously been agreed upon with the patients themselves. It comprised two well-differentiated phases:
- •
Monitoring during the neurostimulator test phase
- •
Monitoring once the final neurostimulator was implanted
In the centre’s initial protocol that had been used since 2018, clinical scales were administered to patients prior to implantation, at 15 days during the test phase, and subsequently at the final implantation. Later on during follow-up it was more irregular, at six and 12 months. If the test phase was unsuccessful, the last questionnaires were not administered and the implants were removed. The questionnaires used, taking account of recommendations from the Spanish Pain Society (SED) for chronic pain, were the following:
- •
DN-4 [Dolor Neuropático 4] for neuropathic pain
- •
Visual analogue scale (VAS)
- •
Oswestry Disability Index for low back pain (ODI)
- •
Patient-perceived satisfactory improvement scale (between 0 and 10)
During the adaptation of the protocol, the experts decided to incorporate new questionnaires to complement the previous ones. These were the following:
- •
36-Item Short Form Survey quality of life scale (SF-36)
- •
Neck Disability Index (NDI): for those cases in which the ODI scale was not applicable due to it being cervical involvement
- •
Patient-reported experience measures (PREMs): to evaluate the experience, satisfaction and usability of the application
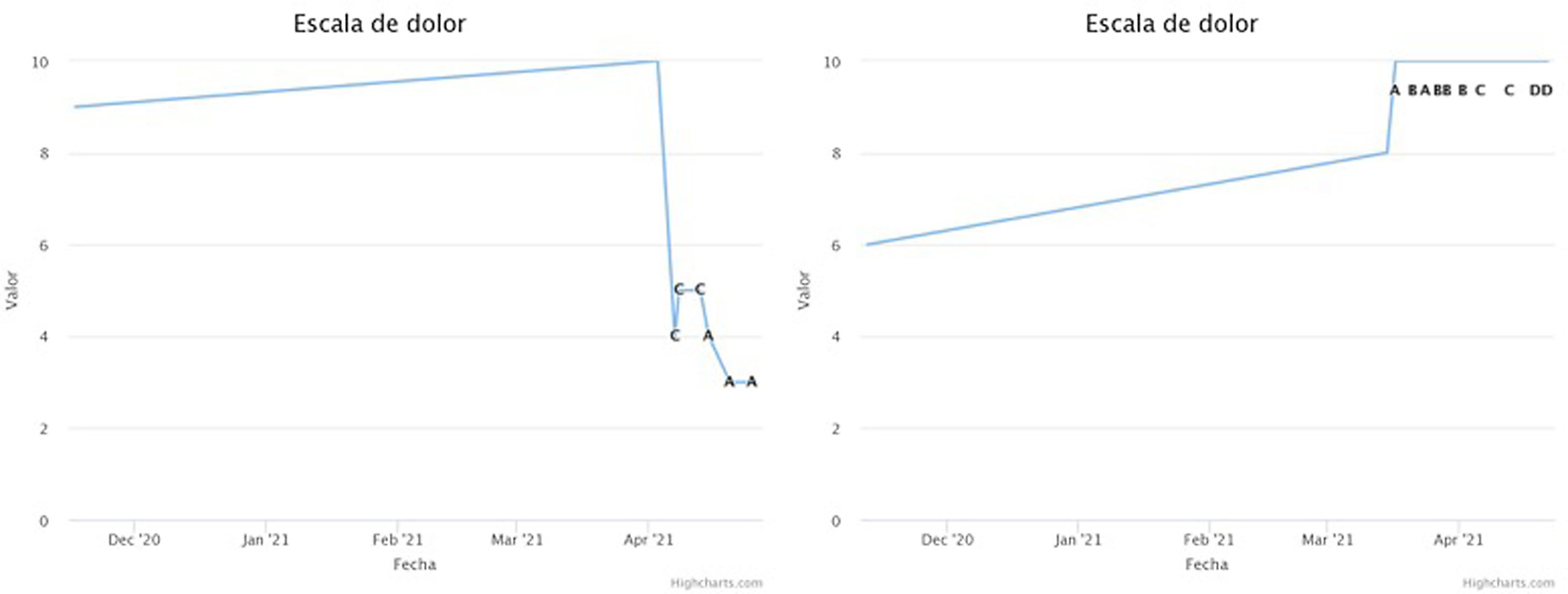
All the questionnaires described above were administered to the patient following a defined protocol. The frequency of sending the questionnaires, the type of questionnaire and the window of availability for its completion varied depending on the phase the patient was in. The results obtained in the test phase were focused on assessing the improvement of the patient's pain and his/her functionality. In this way, by means of integrated monitoring, the final result could be obtained completely remotely (Fig. 1).
Example of graphs obtained from the application during the follow-up of two patients, covering the period before implantation and the test phase. The numerical value of the VAS is used. On the left is a patient with pain improvement during the test phase, and on the right is a patient not only without improvement but with worsening. The graphs show the dates and the type of programming that the patient is using at all times (from A to D).
Once the patient had undergone surgery for implant of the final generator, the remote follow-up phase of the final phase began. In this phase, the questionnaires were distributed either monthly, quarterly or half-yearly, with a scope of up to two years of follow-up in the final phase, which could be modified depending on the specific needs of the patient. The questionnaires that the patient completed throughout the programme were able to generate visible alerts for the clinical team that could indicate a possible worsening of the patient’s clinical course.
Adaptation of the technological platform to the clinical protocolFor the development of the integrated solution, the technological platform used by Medtronic in other patient monitoring projects was taken as a reference. The technological platform in question has CE-Class I marking, with the necessary legal requirements to manage sensitive and especially protected data in the clinical field.
The proposed solution was composed of three basic pillars: a mobile application for patients, a website for the clinical professional and the RPMSC.
Mobile application for patientsThe mobile application was an intuitive tool developed for patients with chronic pain and was compatible with both iOS® and Android® to ensure the widest possible acceptance by patients.
An invitation was required to access it, which was sent to the patient via different channels such as e-mail and SMS. After creating their own credentials, the patient could access the following functionalities:
- -
Clinical data
- -
General documents of interest to the patient (e.g., informed consent, etc.)
- -
Educational material with specific training on the patient’s condition and therapy
- -
Access to a workshop for the management of chronic pain
- -
Specific follow-up questionnaires to assess the patient's clinical course
- -
Event reporting
- -
Calendar and history of scheduled tasks for patients (depending on the phase of therapy they were in)
- -
Automatic and manual notifications
- -
Technical support in the event of issues with the mobile application
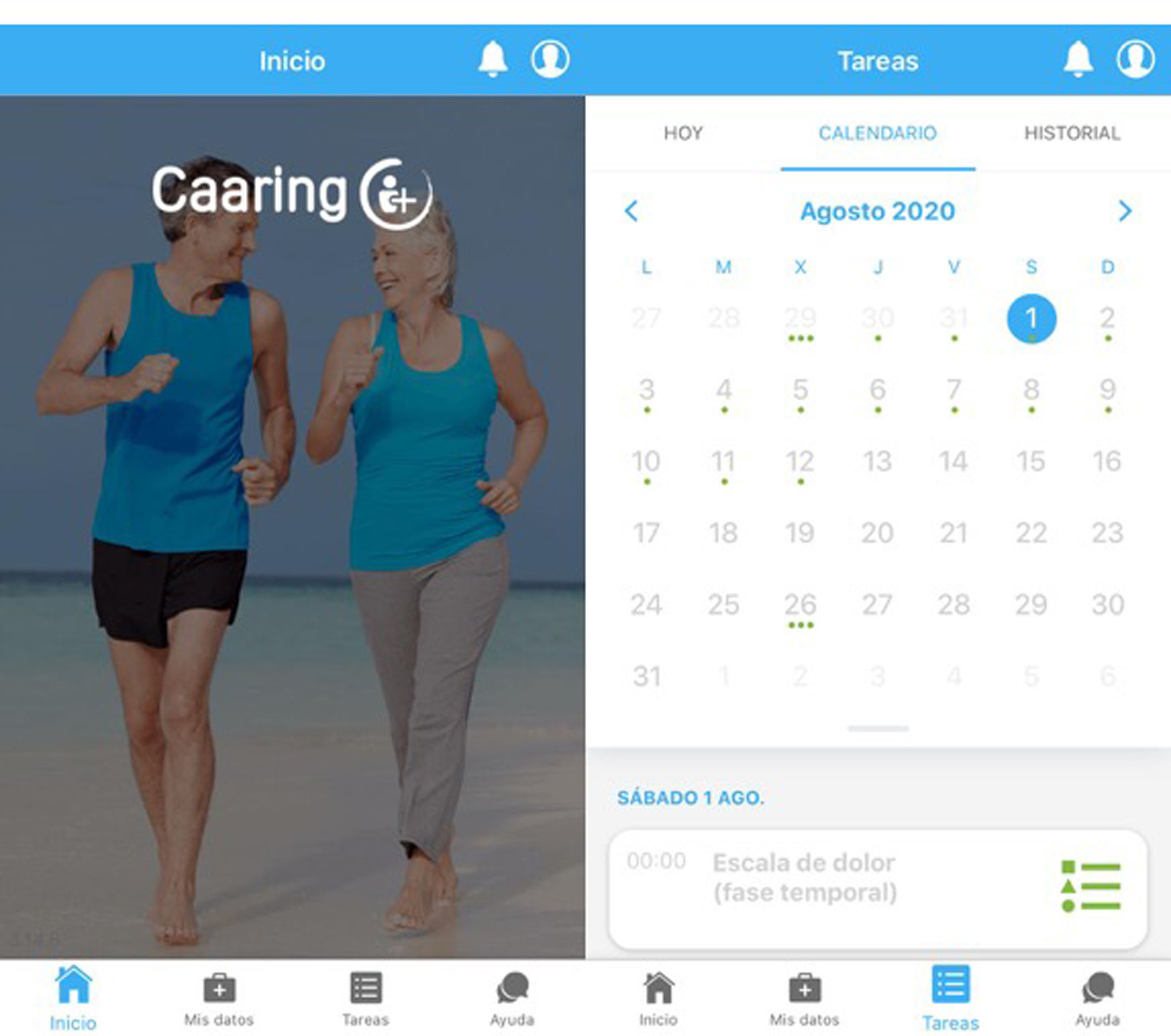
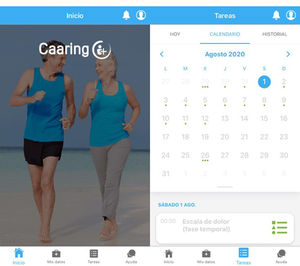
An example of the application can be seen in Fig. 2.
Screenshot of the application’s home screen (left-hand side), with the different access points. Example of the tasks section (right-hand side), featuring a calendar with upcoming activities marked with a green dot, so that selecting a specific day allows you to check what task will be done that day. The “Caaring®” mobile application was developed by Persei vivarium S.L.
The web platform developed for clinical professionals by the company Persei vivarium S.L. aimed to provide information of clinical value to facilitate patient follow-up. The web page could be opened from any browser on any computer inside or outside the hospital.
In addition to the specific information about each patient (demographic and clinical data), the platform included access to a control panel only visible to the healthcare professional to help manage the different alerts. Validation of the alerts was defined by the multidisciplinary group of experts. Alerts were classified by colour:
- -
Red: clinical care was required for a surgical wound infection
- -
Yellow: of clinical consideration due to possible deterioration in the patient’s clinical course
- -
Blue: alert for a warning about generator depletion
The RPMSC was made up of a group of external professionals whose main objective was to increase the efficiency of the healthcare process and improve health outcomes. To achieve this, it performed certain tasks that allowed healthcare professionals to dedicate themselves to patient care.
It was responsible for implementing the programme and for achieving maximum adherence by patients, as well as providing technical support to the professionals involved, by facilitating adaptation by the hospital centre and by the patients themselves. Lastly, it was there to help with data analysis, upon request from the hospital and/or on a regular basis.
Legal considerationsBeing considered a technological platform with CE-Class I marking, compliance with the mandatory legislation regarding essential requirements to manage specially protected clinical data was ensured.
Additionally, various legal agreements between the hospital centre (data controller) and Medtronic (data processor) were required, in order to comply with the provisions of Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data. Medtronic is the bearer of all rights of the solution, and it can therefore exclusively exercise the rights to reproduction, distribution, public communication and transformation.
The solution is intended for healthcare professionals, but under no circumstance does it replace doctor or nursing care. It was used as a complementary tool to help monitor patients, such that, should any issue of concern arise, the patient was redirected to contact his/her doctor through the usual channels. In addition, for patients to access the integrated care programme, they had to sign a dedicated informed consent form, giving their express consent to the processing of their data.
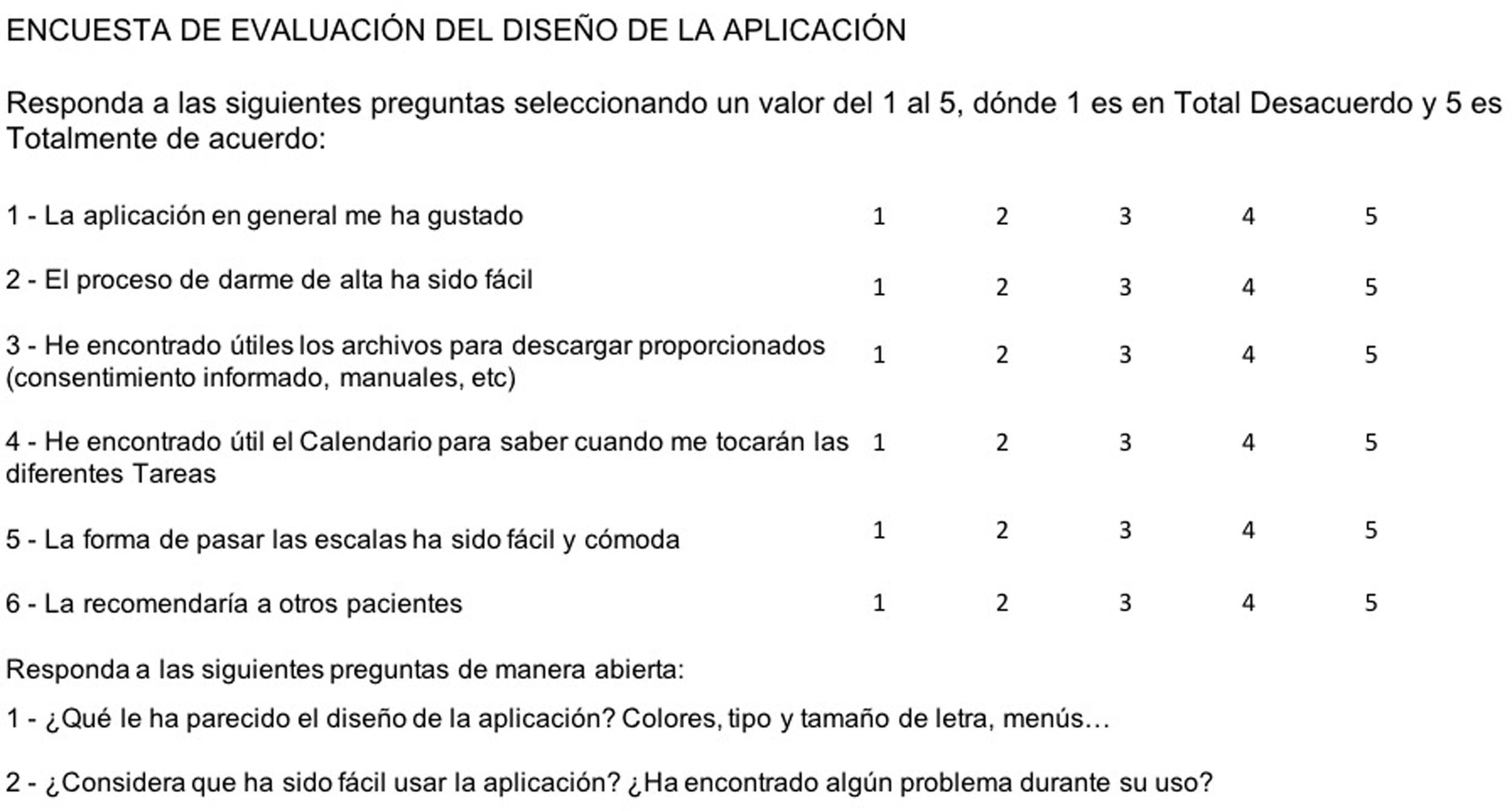
User experience assessmentAlongside the various questionnaires, a user satisfaction and experience assessment survey was developed for the patients and healthcare professionals involved (Fig. 3).
This survey focused on aspects of the programme’s design. Additionally, a question was added about the general satisfaction of the user and another related to the Net Promoter Score, indicating whether the user would recommend this application to other patients. In the final section, open questions were formulated to encourage interaction. These surveys were administered by telephone to patients who had completed the test phase and had the final generator implanted, having gone through all the phases available in the application.
ResultsAfter completing all the steps described above and obtaining the relevant hospital legal authorisations, the integrated solution was implemented in October 2020. From that moment on, patients who were on the waiting list for an implantable neurostimulator began to be included from consultations. However, due to the COVID-19 pandemic, the implantation of neurostimulators was not permitted until March 2021.
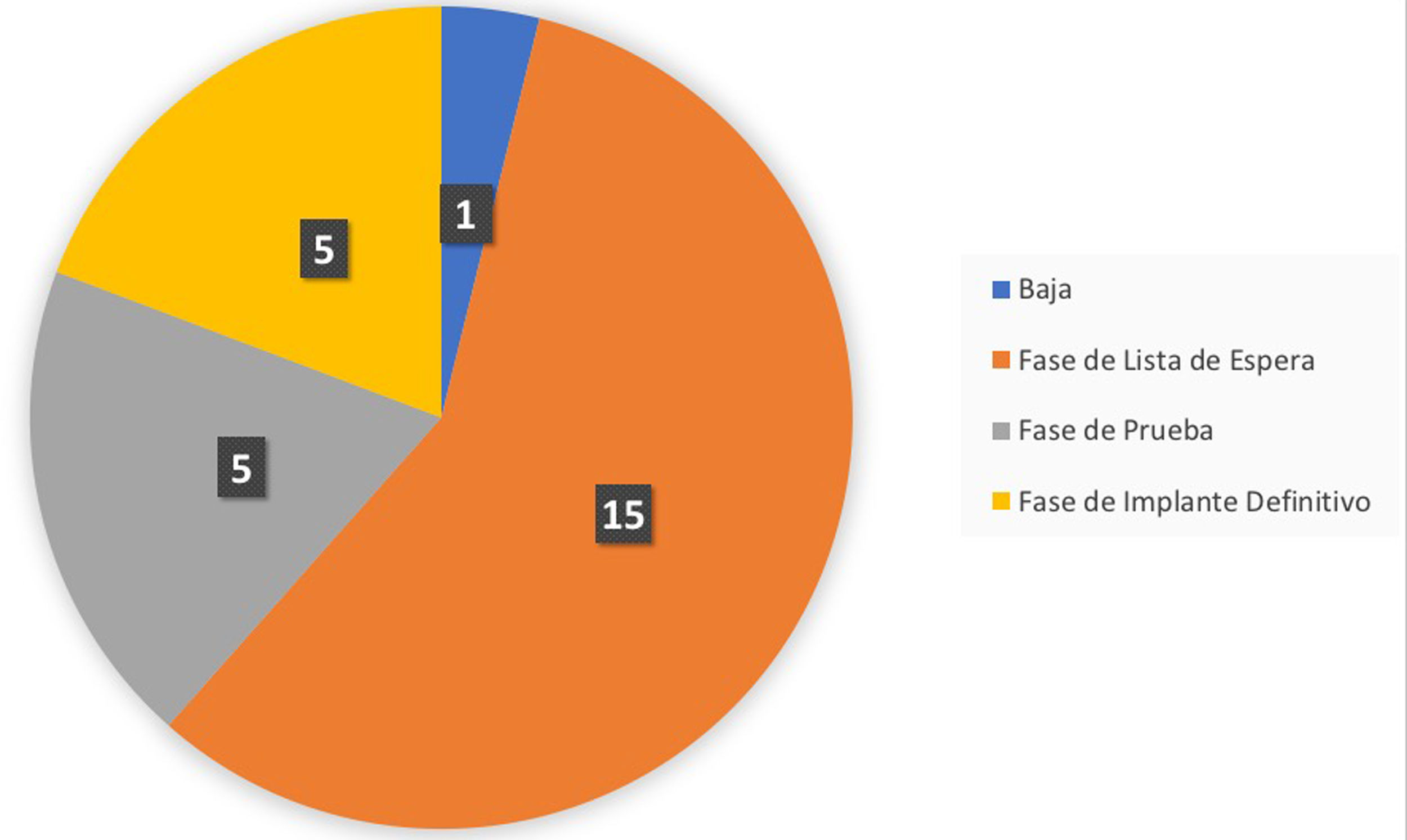
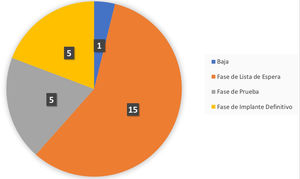
Up to the export date (April 2021), a total of 26 patients were included in the follow-up care programme, of which four (15%) were in the test phase and six (23%) had completed it. Of those six patients who had completed the test phase, in five (83%) of them the result after passing the test phase was positive and the final generator was implanted, so they moved on to the final phase in the follow-up programme. In the remaining case (17%), the system was withdrawn because the test phase was considered to be negative (Fig. 4).
Summary of the phase of the integrated care programme for patients with chronic pain and an implanted neurostimulator the patients are in, with an extraction date of 21 April 2021. Specifically, the graph indicates the following states: Withdrawn: patients who have a test phase with an unsatisfactory result; Waiting list phase: patients who are on the waiting list for the implantation of the test neurostimulator; Temporary phase: patients who are in the test phase; Final phase: patients who have passed the test phase with a satisfactory result and have had the final generator implanted.
In total, 230 questionnaires have been administered to date, 126 of which correspond to before the test phase, 98 during the test phase and one from the final phase. Additionally, one warning was received due to low battery of the generator in the test phase and five surveys to evaluate the design of the programme were conducted. These data represent an average of 8.85 questionnaires per patient to date (Table 2). The total number of actions required by the RPMSC, understood to be contacts with the patient through app or SMS notification, was 52. The ratio between the number of patients and the number of contacts was 1:2. The percentage of adherence was 82% in patients before the test phase, 75% in the test phase and 100% in the final phase.
Forms distributed by the application.
| Type of form | n (m) |
|---|---|
| Infected wound | 0 |
| Low battery | 1 |
| Pain VAS | 114 |
| ODI Questionnaire | 17 |
| NDI Questionnaire | 21 |
| SF-12 Questionnaire | 36 |
| DN-4 Questionnaire | 31 |
| Patient-perceived satisfactory improvement | 5 |
| Patient experience | 1 |
| Programme design evaluation | 5 |
| Total (m) | 230 (8.85) |
Summary of the data recorded in the application dated 21 April 2021. The total number of forms filled out by patients is indicated according to the type.
VAS: visual analogue scale; n: number of patients; m: mean.
The first five patients who had the final generator implanted were selected to complete the programme design evaluation survey. This survey was completed via a telephone call with a member of the group of experts. The survey revealed that the sign-up process and completing the scales was rated favourably, while the files to download and the calendar were less well accepted. In the case of downloads, patients reported that they did not know where to find these documents. Regarding the calendar, they reported that they used it a lot because a notification alerted them to the fact that the test was available. Finally, overall, the application’s score was favourable and they would recommend it to other patients. The results of the numerical part can be seen in Table 3. In the free writing section, of note was the fact that one patient reported that he did not like the range of colours and another said that the font was too small. In one of the cases, it was reported that the patient did not fully understand the RPMSC contact in the event of not responding to a task. There were no serious issues regarding application usage.
Evaluation test results.
| Question | Patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | m | |
| 1 - I liked the application overall | 3 | 4 | 5 | 4 | 4 | 4 |
| 2 - The sign-up process was easy | 4 | 5 | 5 | 3 | 5 | 4.4 |
| 3 - I found the downloadable files provided useful (informed consent, manuals, etc.) | 1 | 3 | 4 | 2 | 3 | 2.6 |
| 4 - I found the Calendar useful for seeing when the different Tasks are scheduled | 1 | 3 | 2 | 2z | 3 | 2.2 |
| 5 - Doing the scales was easy and convenient | 4 | 4 | 5 | 3 | 5 | 4.2 |
| 6 - I would recommend it to other patients | 3 | 4 | 5 | 4 | 5 | 4.2 |
Data collected from the programme design evaluation questionnaires with the results given by each patient individually. The results of this survey range from a score of 0 - “Strongly Disagree” to 5 - “Strongly Agree”.
m: mean.
Taking account of the results of the survey, adjustments have been made to the application. To solve the problem of downloading files, an alert system has been programmed so that when one of these documents is uploaded to the application, the patient receives a notification directly to his/her mobile. As for the range of colours and font, work is being done to offer various possibilities regarding the visual environment.
DiscussionOver the last few years, a large number of pain-related mobile applications and digital solutions have appeared for purposes such as postoperative follow-up, self-management of pain or to control chronic pain directly8–10. However, many of them are not sufficient or did not meet expectations. The design and the way they approach the problem has not been executed with adequate prior scientific study that would foresee success later on6, although in some cases they do seem to be useful when compared with classic or traditional follow-up10. The main difference between these digital solutions and the one we have designed in our hospital is in the existence of a support centre that is responsible for monitoring the follow-up. This in itself is a novelty and, to the best of the authors’ knowledge, is not widespread around the world. Therefore, having an operational support centre constitutes the main strength of the project and is what makes this study original.
In a recent study in 19 European countries, the prevalence of cervical/lumbar pain was found to be 40%11, and this is associated with a high demand for medical resources, without significant improvement in treatment outcomes12. In addition, the need to maintain continuity of care for these patients at times such as during the COVID-19 pandemic, which limits face-to-face consultations13, is fundamental. For patients with a spinal cord stimulator, there is the added disadvantage of the need for more appointments to programme and assess the devices. This is why the urgent need for a viable remote health programme for patients with these systems, that allows for optimal follow-up, has been raised in other areas of neuromodulation14. The application designed at our department, along with help from the support centre, makes it possible to reduce the influx of visits to the hospital, with the patient feeling that they are being adequately monitored. The different tasks and the additional materials available help with adherence, but the system also has a new alert system that informs the team of professionals of events as important as wound infections or the depletion of generators. It has also been proven that the use of a mobile application is an additional advantage for those patients less able to express themselves or who are more reluctant to talk about their condition15, since the tasks are carried out without pressure, in a friendly environment, without time constraints and with the greatest of privacy.
Many and diverse mobile applications have appeared in recent years specifically aimed at people with pain, whether related to cancer or not16. Many of these have been openly marketed on the main digital platforms, accessible to anyone who would like to download them. However, after analysing them, it is clear that the majority (approximately 86%) have not been developed with professional medical participation17. Lallo et al.18 reviewed 1,019 applications, looking for those with the stated objective of providing education, tools or advice related to pain management after surgery, and found that only 10 met the established criteria. In addition, when they analysed which of them had been developed with healthcare professionals, they identified only five that met this premise (0.49% of all those analysed). Bhatterai et al.17 examined 373 arthritis-related pain self-management applications and found that only four met the Stamford pain self-management programme criteria. In another recent review of 195 pain management applications, Portelli and Eldred19 applied the criteria of cognitive behavioural therapy guidelines to their evaluation of computerised programmes. They found that only six (just 3%) met these guidelines. The authors concluded that existing pain applications have often been created by software developers, but without much participation from healthcare professionals and pain patients themselves. At the same time, they realised that pain applications tend to have minimal theoretical content to facilitate self-management and/or behavioural change. Lastly, Pfeifer et al.20 conducted a meta-analysis of articles related to pain management applications, obtaining a sample of 4,767 patients from 22 valid randomised studies, but pointing out that the quality of these was not optimal, with only eight meeting at least three of the four established quality criteria. Even so, they determined that there is a small but significant long-term impact on pain control in those who used an application for this purpose.
Taking all of the above into account, there is also a decisive factor for a mobile application, and that is its ability to remain active, since the life expectancy of most smartphone applications is short. In total, 75% of users stop using an application within 48 h of downloading it, and 25% of applications are deleted after they are opened for the first time21. For these reasons, the combined use of the application alongside a support centre, together creating an integrated solution, is what can really make the difference. There are few references that concern the use of both factors in combination. Alam et al.15 analysed the use of 3,984 users of a maternity application that has a payment service for telephone consultations, defined as “brief”. They concluded that, in general, users consider the telephone system to be convenient, cost-effective and reliable, and that this service considerably improves the use of the application. Perdoncini et al.22 used video calling from within a specific application to perform oral examinations, with diagnostic results comparable to those of the standard procedure. In similar vein, Jamison et al.23 analysed the impact of using a pain management application with and without the use of text message reminders to perform tasks. They observed that the group with reminders tended to use the application more and send more tasks than those who did not, although the difference in pain control in both groups was not statistically significant. In a very recent study related to the post-hospitalisation follow-up of COVID-19 patients, Shah et al.24 analysed a digital solution that combines the use of a mobile application with a telephone monitoring centre to assess whether the application was able to reduce the flow of calls to this centre. They found that the group that used the application made, on average, half as many calls as the other group. Finally, in another very recent study, Ooi et al.25 compared the follow-up and adherence of patients with facial nerve blocks with a mobile application that performs self-administered questionnaires and a direct telephone call. They analysed 120 patients, 60 in each arm, and concluded that in both cases the percentage of follow-up was low, and that the only advantage of using the application was to reduce the attendance time of medical personnel. In the case of our integrated solution, which included a mobile application, a text messaging system for task reminders and a support centre that made calls and did follow-up, we combined all the facets of what is in the current literature to try to achieve the best possible adherence. As the application itself develops, we will determine whether the implementation of all these measures is sufficient to achieve the goal of maximum adherence.
Regarding the limitations of this project, there are several to take into account. The main limitation is in regard to the type of patient for whom it is intended, since there are not many patients with neurostimulators and they usually have implications inherent to their chronic pain process that condition their way of acting and responding to any intervention that they undergo. Furthermore, and along the same lines, they are patients with a high emotional component (including anxious-depressive clinical signs and symptoms), and this can affect their follow-up. Other limitations include the use of technology by the user, because, although use of mobile phones with functionality for applications is widespread, those generations that have not undergone the technological transition or are not digital natives may encounter difficulties in using it. This is partly mitigated by the support centre, but it is still a limitation. And the final limitation is the great need for resources that are required to start a project like this and the fact that a financial endowment is needed that has to be borne by the health system.
ConclusionsDigital systems as an additional facet of healthcare are not only the future, but are already a reality, and healthcare professionals must play an essential role in their design alongside the specialised industry. With this in mind, the development of a solution for monitoring patients with a neurostimulator for chronic pain is presented, following a creation process based on scientific evidence and in accordance with established protocols. In addition to the patient application and the professionals' web environment, there is also a specialised support centre, creating an integrated solution that ensures greater adherence to follow-up and better patient care.
Conflicts of interestDr Cordero Tous, Dr Santos Martín, Dr Sánchez Corral, Dr Román Cutillas and Dr Horcajadas Almansa declare that they have no conflicts of interest and have not received any remuneration for their work on this project. Ms Nuñez Alfonsel and Ms Román Moyano work for Medtronic Ibérica S.A. (“Medtronic”).
The project was developed as part of the programme for the development of a comprehensive operational framework of the Neurosurgery Clinical Management Department of the Hospital Universitario Virgen de las Nieves [Virgen de las Nieves University Hospital], signed between the Hospital Universitario Virgen de las Nieves in Granada and Medtronic Ibérica S.A.
Please cite this article as: Cordero Tous N, Santos Martín L, Sánchez Corral C, Román Cutillas AM, Núñez Alfonsel B, Román Moyano M, et al. Desarrollo de una solución integrada para pacientes con dolor crónico portadores de neuroestimulador en tiempos del COVID-19: una aplicación para móvil con centro de soporte. Neurocirugia. 2022;33:318–327.