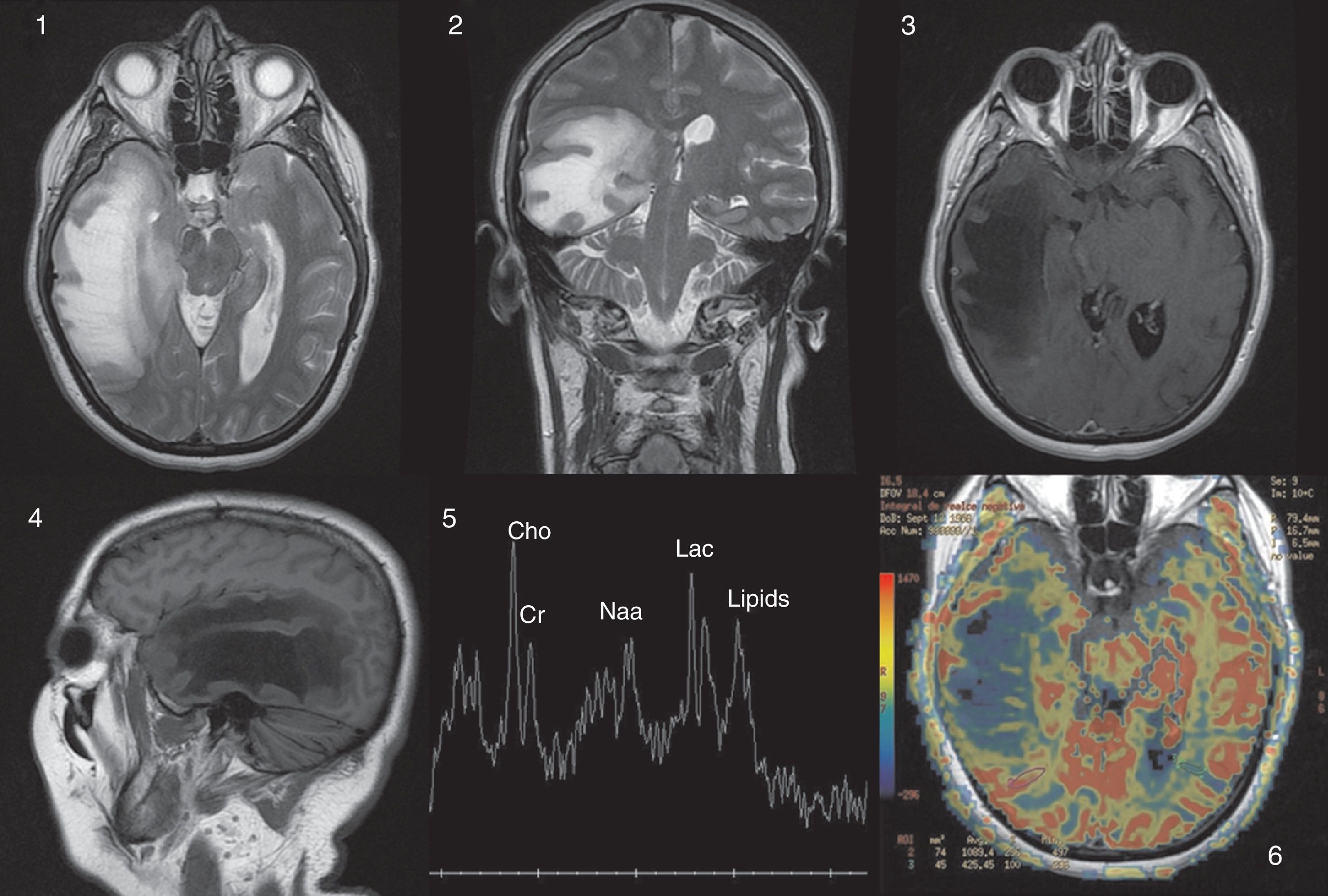
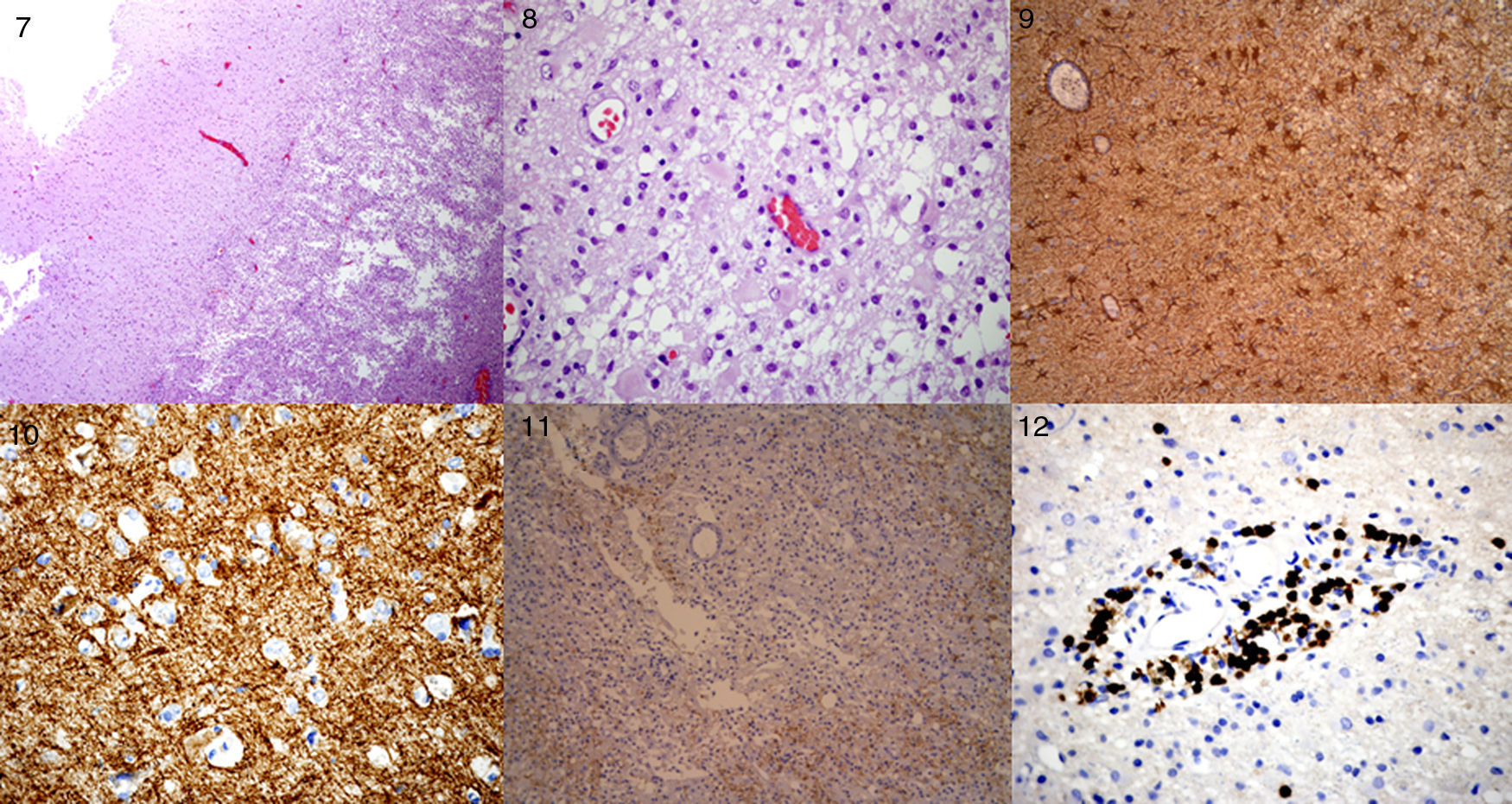
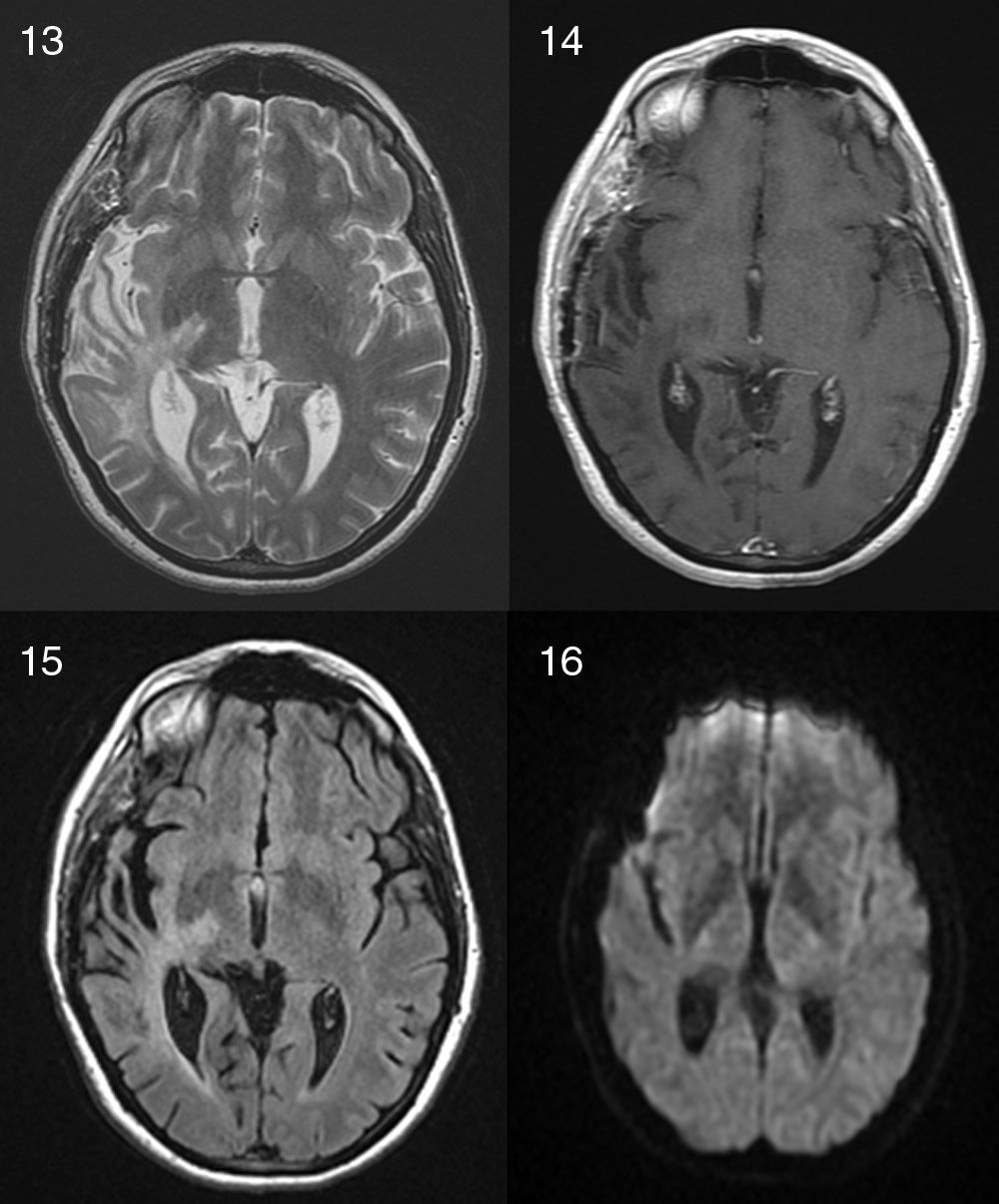
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system, characterized by focal neurological dysfunction with a relapsing and remitting course. Tumor-like presentation of MS (or “tumefactive”/“pseudotumoral” presentation) has been described before with a certain frequency; it consists of a large single plaque (>2cm) with presence of edema and mass effect and it is hard to distinguish from a brain tumor. However, we present a very rare case of a 53-year-old woman with a right temporal mass that turned out to be a MS plaque, who deteriorated within hours (brain herniation with loss of consciousness and unilateral mydriasis) and required an emergency craniotomy. We also present a review of the literature. It appears that only 4 cases of emergency craniotomy/craniectomy required in a patient with a tumor-like MS plaque have been reported before.
La Esclerosis Múltiple (EM) es una enfermedad desmielinizante del sistema nervioso central, que se caracteriza por déficits neurológicos con un curso recurrente-remitente. La presentación pseudotumoral de la esclerosis múltiple ha sido descrita en la literatura con cierta frecuencia, se define como la presencia de una única placa desmielinizante grande (>2cm) con edema asociado y efecto de masa, que puede confundirse fácilmente con un tumor cerebral entre otros diagnósticos alternativos. Presentamos el caso de una mujer de 53 años con una lesión en el lóbulo temporal derecho que resultó ser una placa de desmielinización aguda, la singularidad del caso reside en que la paciente deteriora neurológicamente en pocas horas (coma y midriasis ipsilateral) requiriendo una intervención quirúrgica urgente. En la revisión de la literatura que hemos llevado a cabo, solo hemos encontrado publicados otros cuatro casos en los que un paciente con una placa pseudotumoral de EM precisase una craneotomía/craniectomía urgente.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.