To describe the radiological characteristics, surgical indications, procedures, and intracranial pressure monitoring of a representative cohort of severe traumatic brain injury (sTBI) cases collected over the past 25years, and to analyse the changes that have occurred by dividing the period into 3 equal time periods.
MethodsAn observational cohort study was conducted on consecutive adult patients (>14years of age) with severe closed TBI (Glasgow Coma Scale score [GCS]≤8) who were admitted during the first 48h after injury to the Hospital 12 de Octubre from 1987 to 2012. The most relevant radiological findings, surgical procedures, and intracranial monitoring indications reported in the literature were defined and compared in 3 equal time periods (1987–1995, 1996–2004, and 2005–2014).
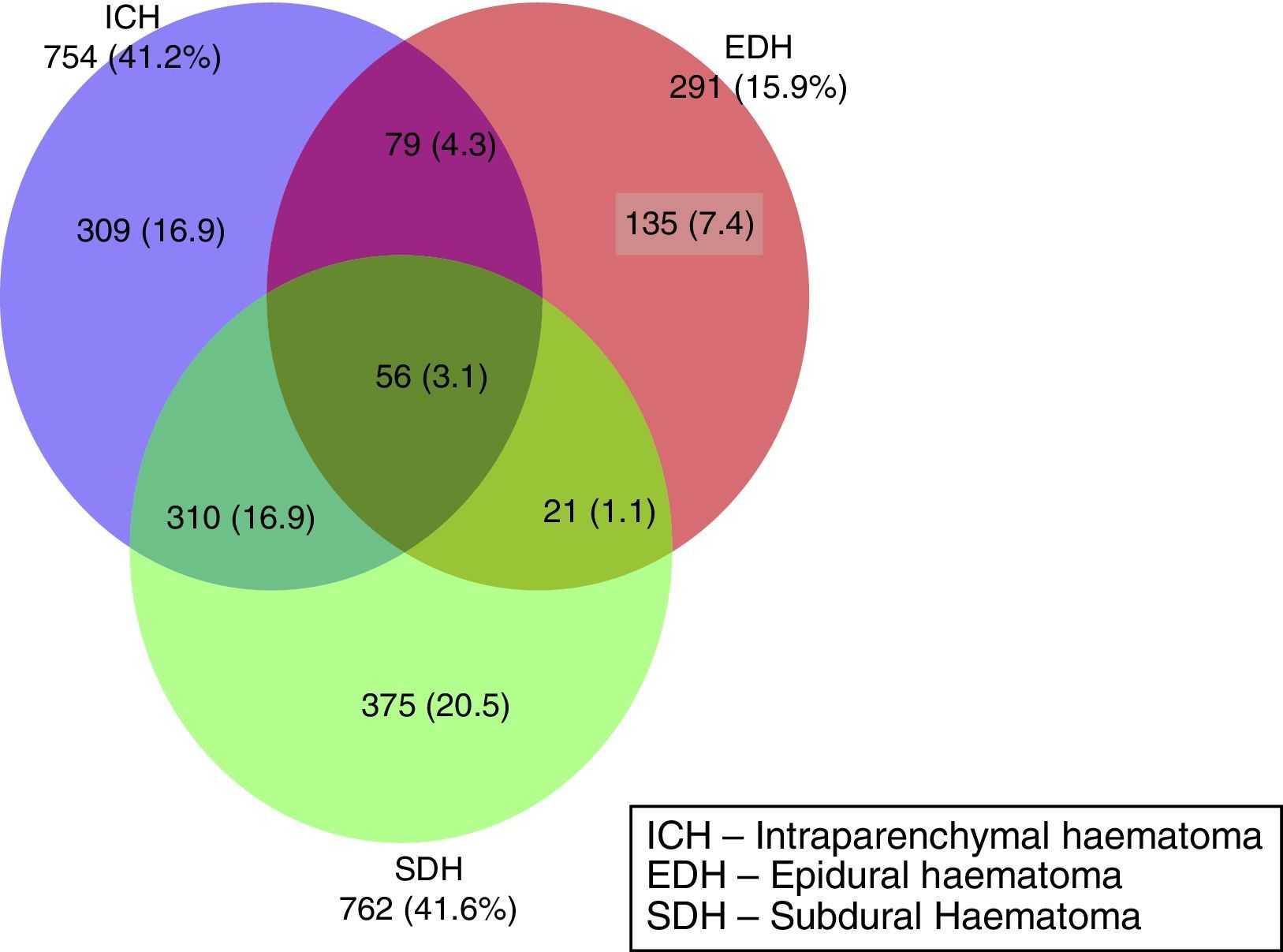
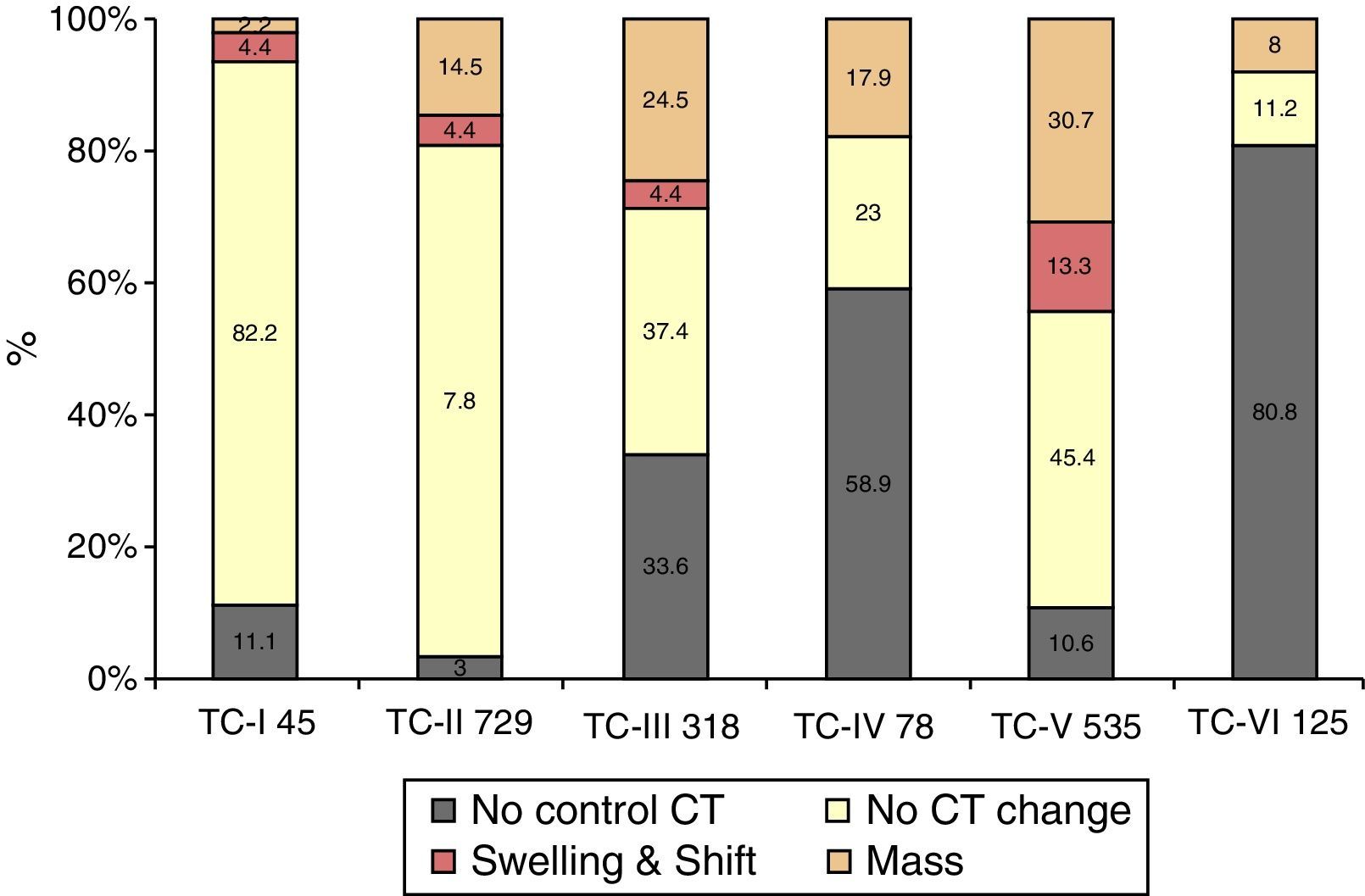
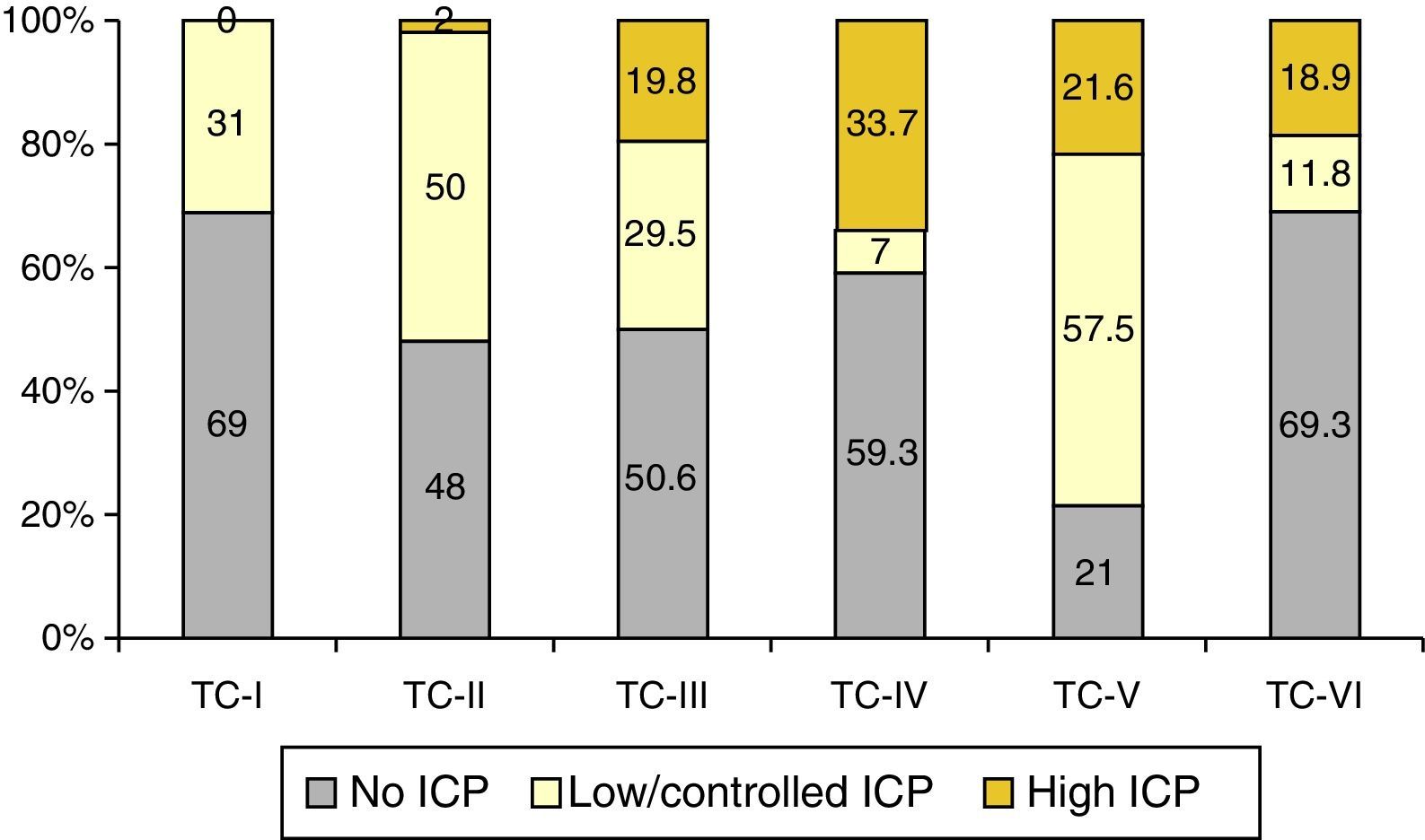
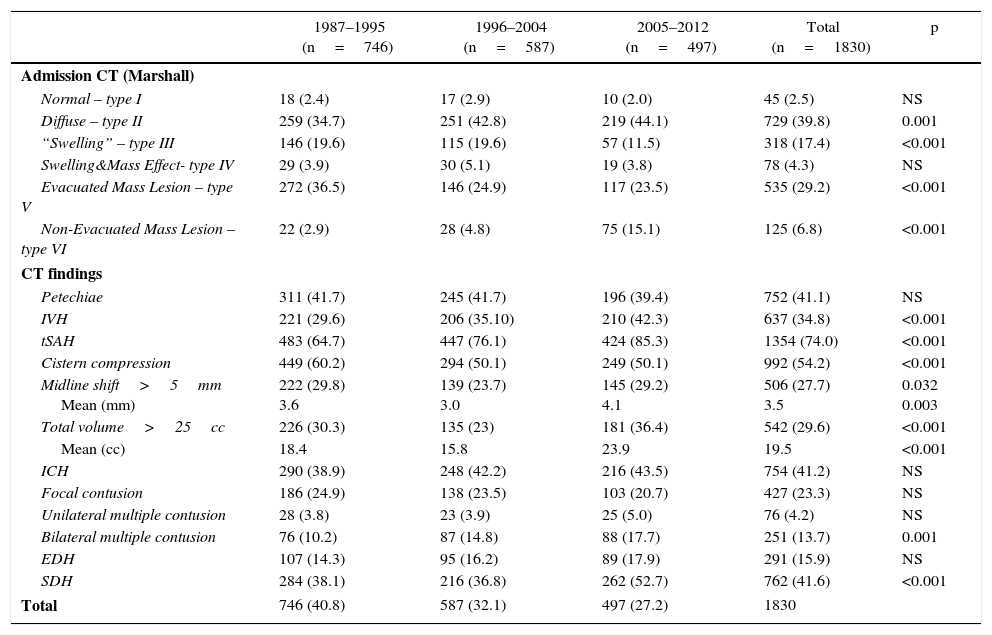
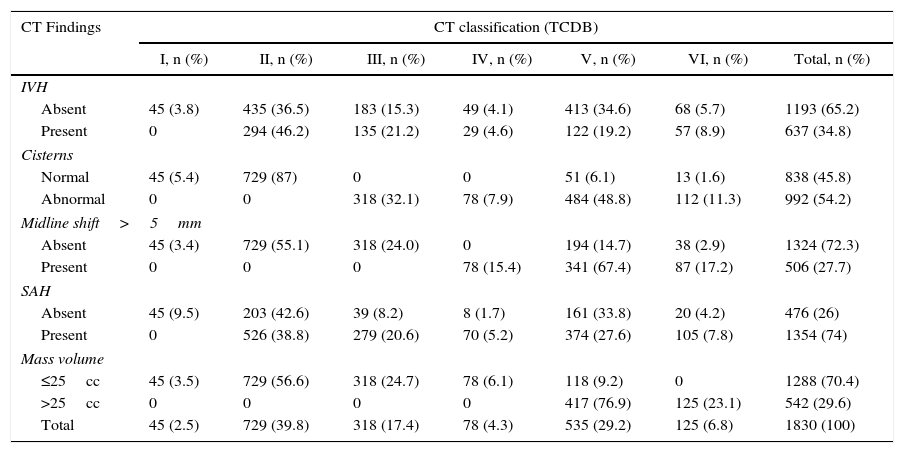
ResultsA significant increase was observed in subdural haematomas with lesions over 25cc, and midline shift in the last period of time. The incidence of subarachnoid haemorrhage increased significantly with time. There was a progression to a worse computed tomography (CT) classification from the initial CT scan in 33% of cases.
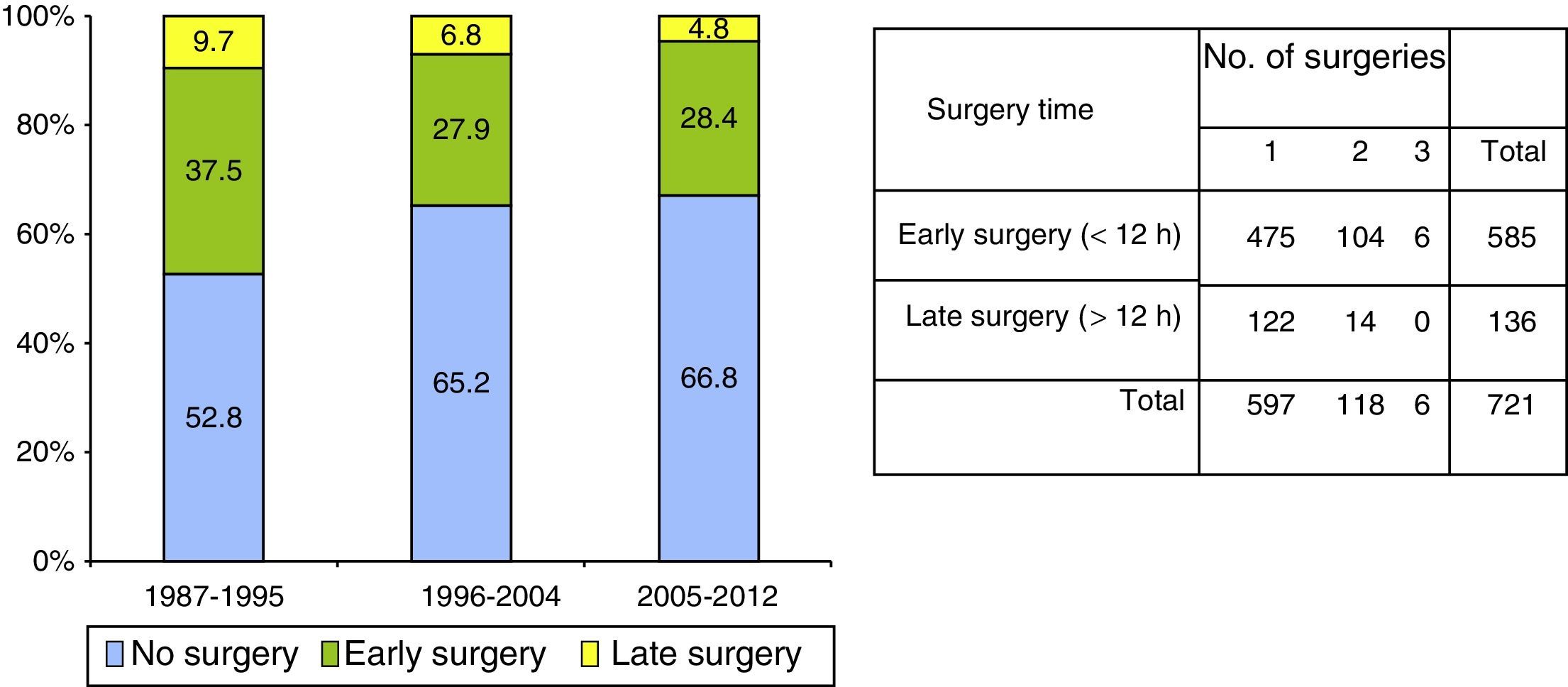
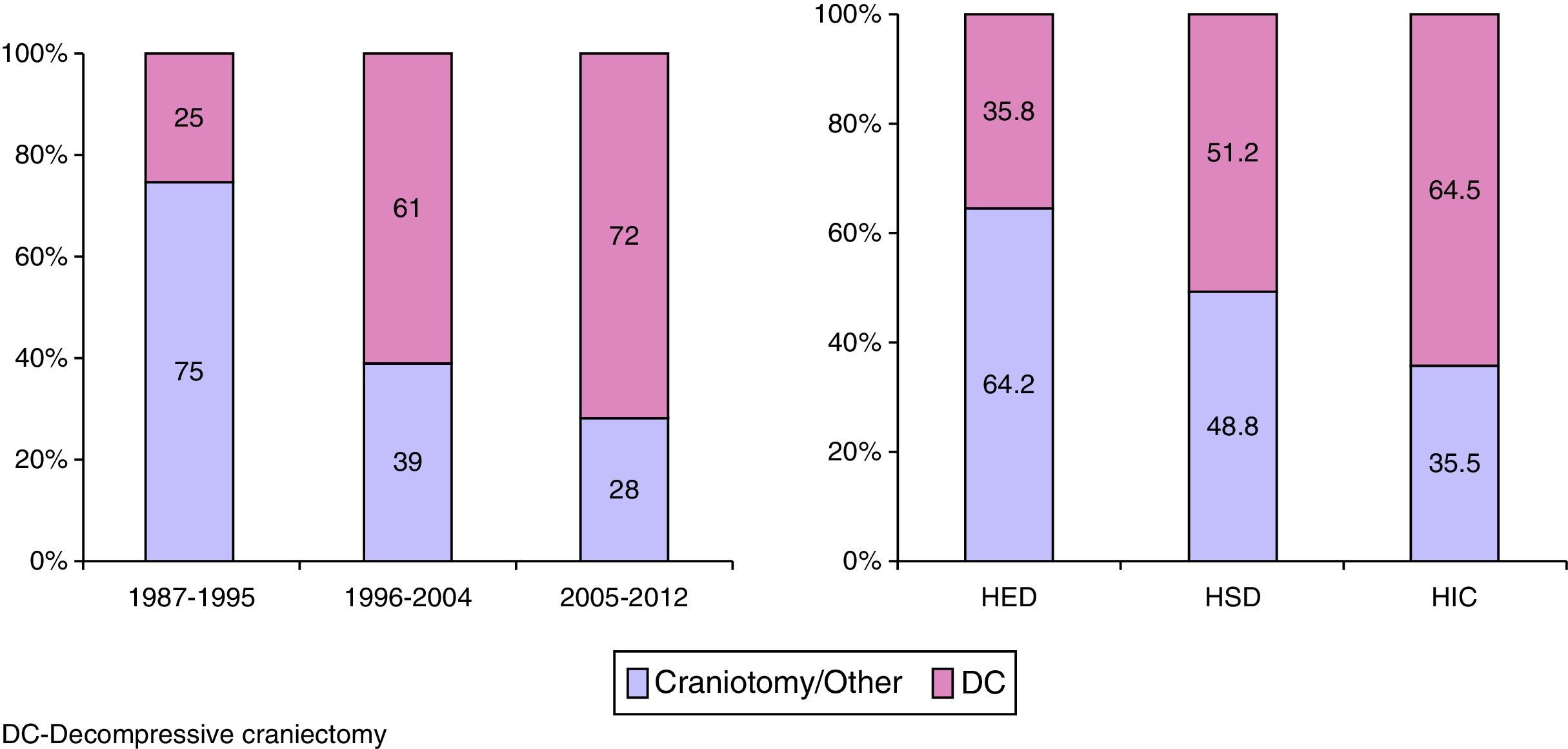
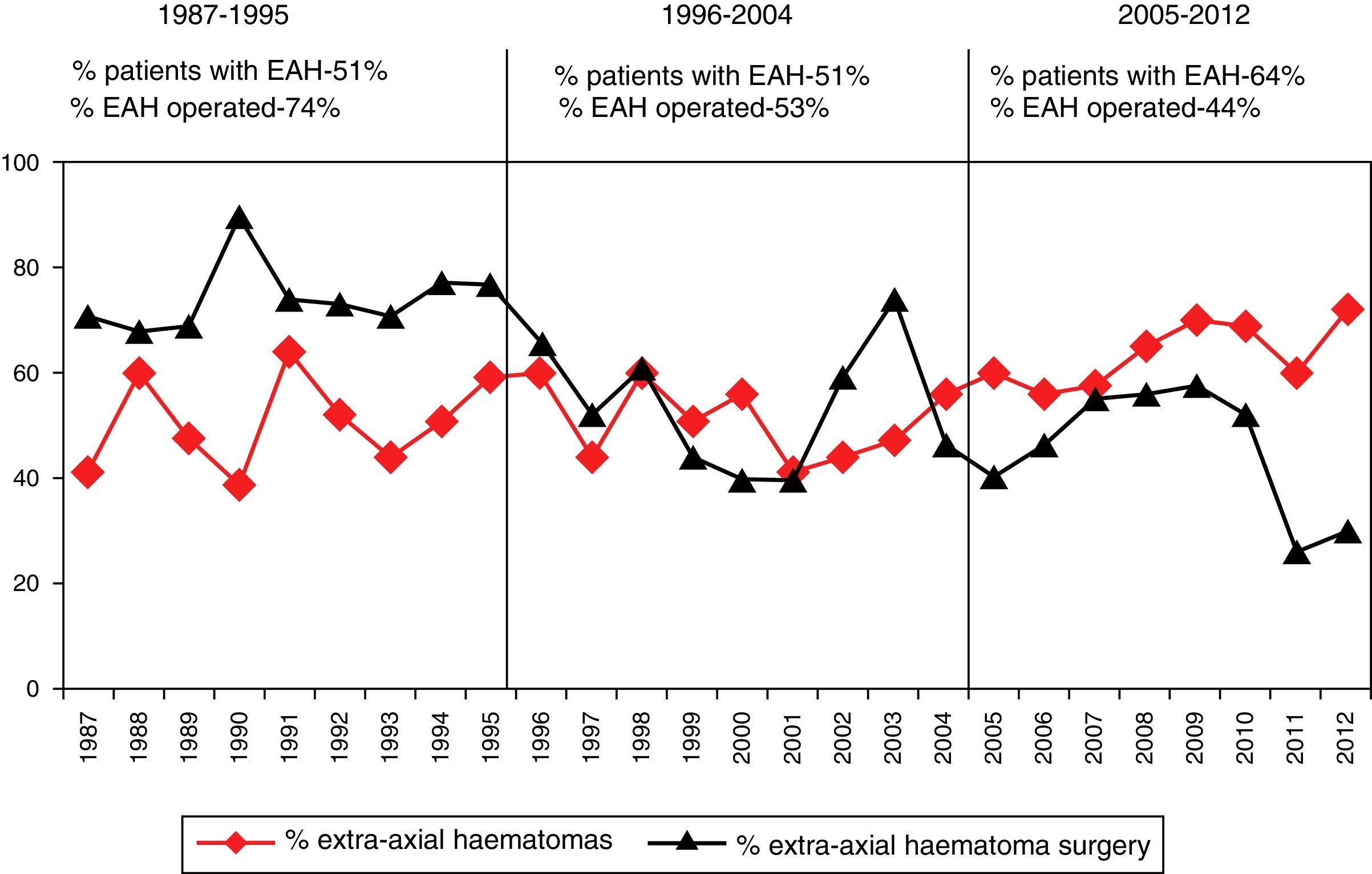
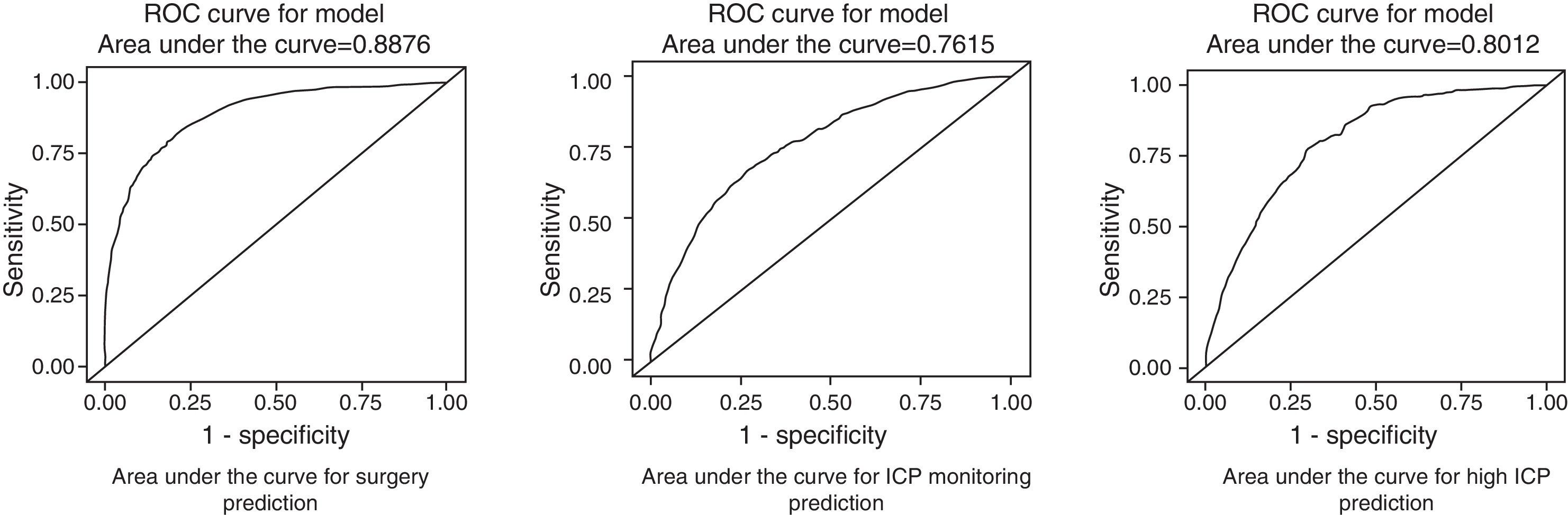
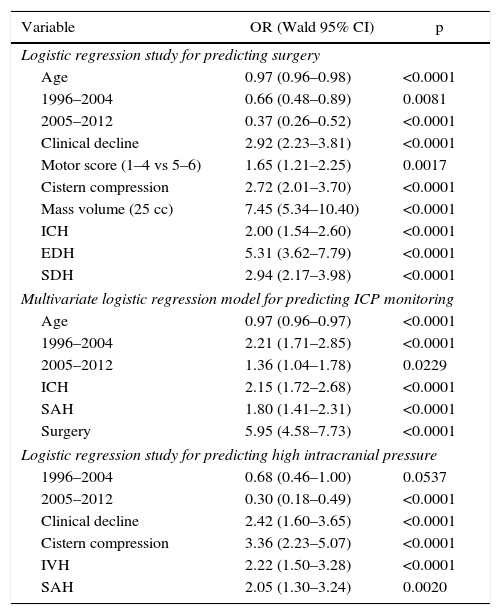
Surgery was performed on 721 (39.4%) patients. Early surgery (<12h) was performed on 585 (81.1%) patients, with the most frequent being for extra-cerebral mass lesions (subdural and epidural haematomas), whereas delayed surgery (>12h) was most frequently performed due to an intracerebral haematoma. Surgical treatment, both early and late was significantly lower with respect to the first time period. Decompressive craniectomy with evacuation of the mass lesion was the preferred procedure in the last time period.
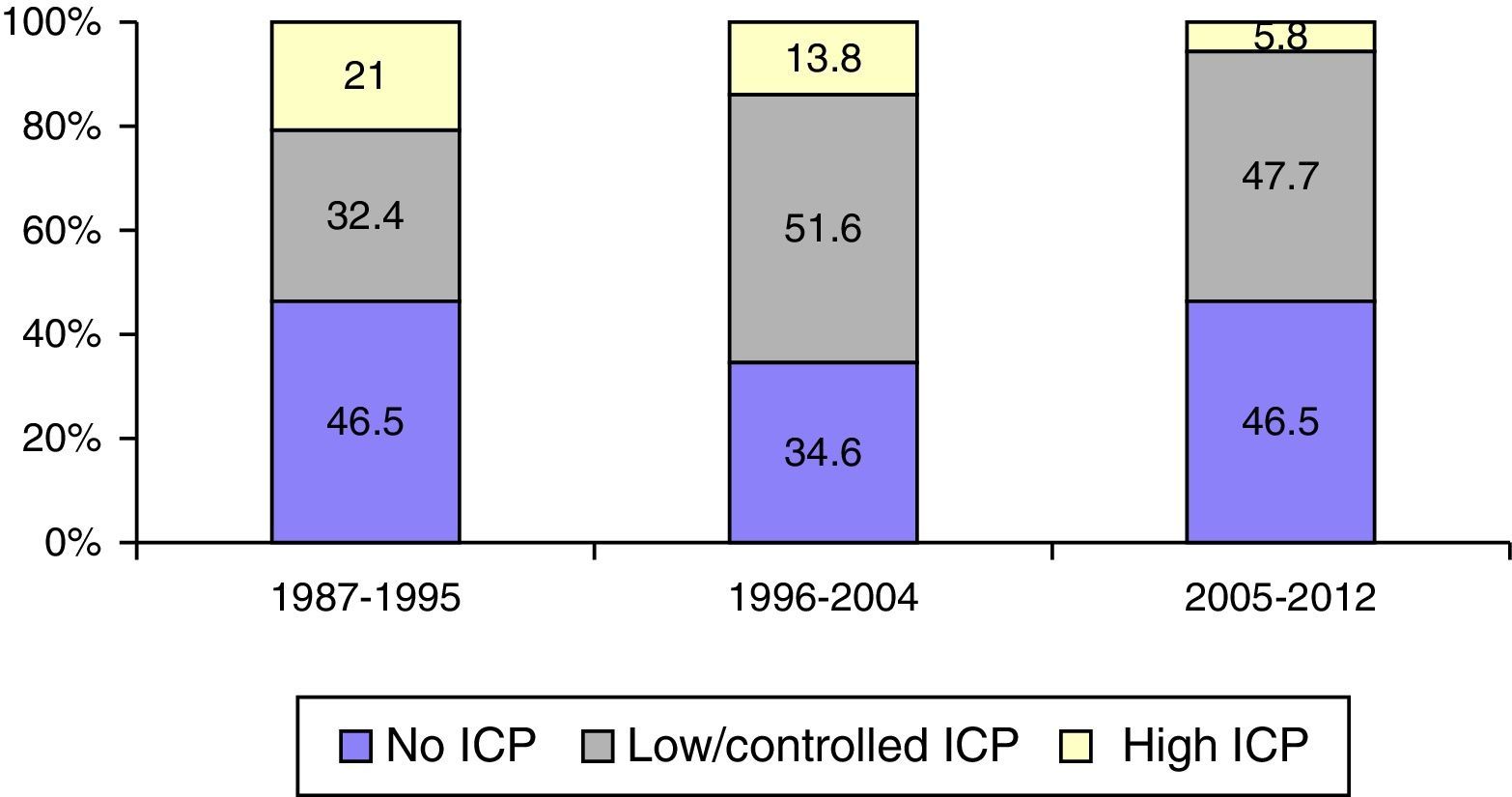
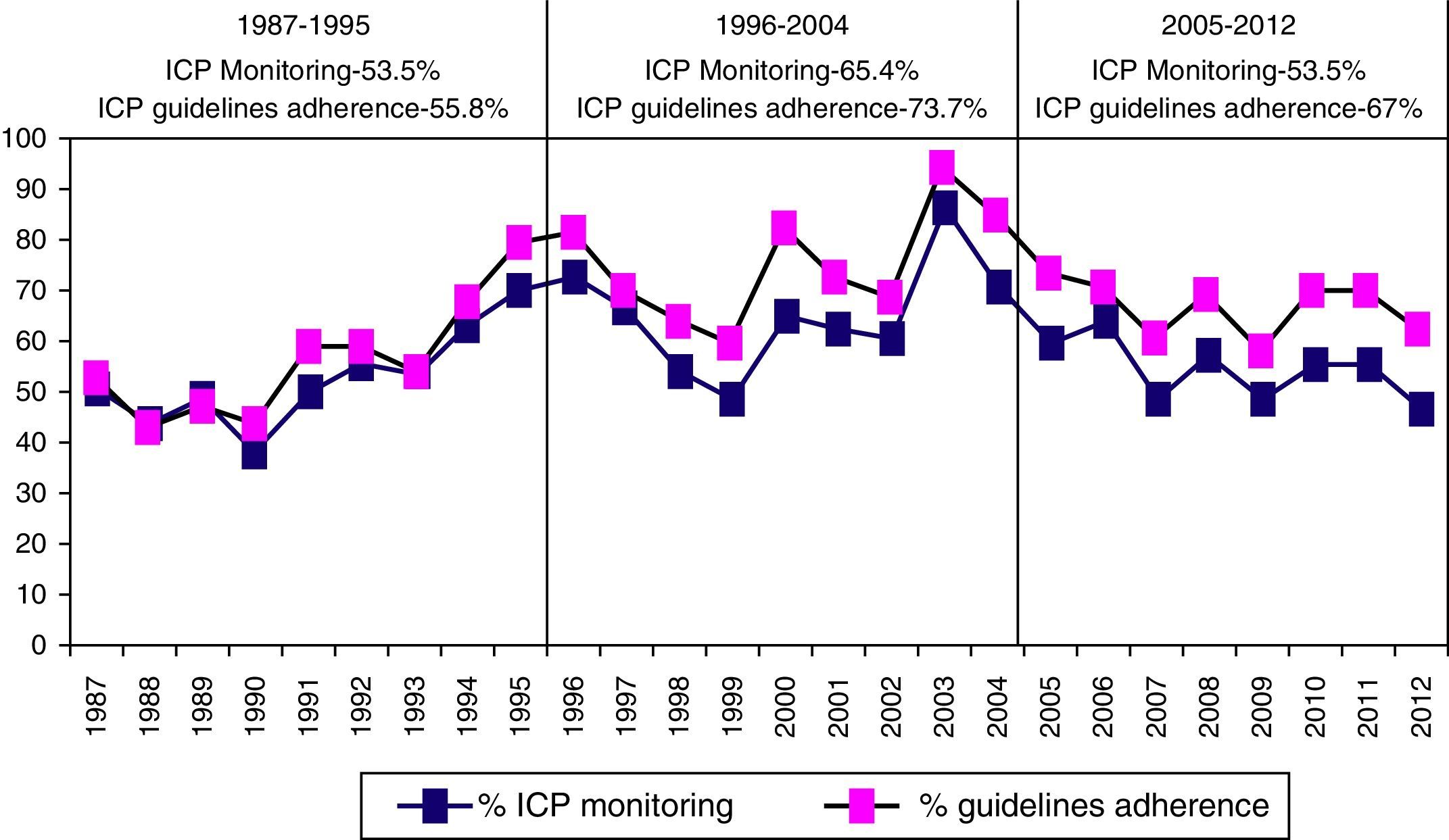
Intracranial pressure monitoring (ICP) was carried out on 1049 (57.3%) patients, with a significantly higher frequency in the second period of time. There was adherence to Guidelines in 64.4% of cases. Elevated/uncontrolled ICP was more significant in the first time period.
ConclusionsAs a result of the epidemiological changes seen in traumatic brain injury, a different pattern of morphological injury is described, as depicted in the CT, leading to a difference in practice during this period of observation.
Describir las características radiológicas, quirúrgicas y manejo de la presión intracraneal (PIC) de una cohorte de pacientes con traumatismo craneal grave (TCEG) ingresados en los últimos 25años.
MétodosEstudio observacional de una cohorte consecutiva de pacientes adultos (>14años) con TCEG cerrado (GCS≤8) admitidos en las primeras 48h del TCEG en el Hospital 12 de Octubre entre 1987 y 2012. Se definieron las características radiológicas, los procedimientos quirúrgicos y las indicaciones de monitorización de la PIC y se compararon en los 3 periodos de tiempo (1987-1995, 1996-2004 y 2005-2014).
ResultadosSe apreció un aumento significativo del hematoma subdural mayor de 25cc, de la desviación de la línea media y de la hemorragia subaracnoidea (HSA) en el último periodo de tiempo.
Fueron intervenidos 721 pacientes (39,4%); 585 (81,1%) en las primeras 12h (cirugía precoz). El tratamiento quirúrgico disminuyó significativamente en el último periodo de tiempo, siendo la craniectomía descompresiva (CD) con la evacuación de una masa intracraneal el procedimiento más utilizado en el este periodo.
Se monitorizó la PIC en 1.049 pacientes (57,3%), con una frecuencia significativamente mayor en el segundo periodo, con una adherencia a las Guías del 64,4%. La PIC elevada incontrolable fue significativamente mayor en el primer periodo de tiempo.
ConclusionesComo consecuencia de los cambios epidemiológicos que se han apreciado en los pacientes con TCEG en los últimos 25años, describimos un patrón diferente de lesión morfológica, como se puede apreciar por el cambio en la TC, lo que determina un cambio en la práctica clínica durante este periodo de observación.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.