To conduct a survival study and evaluation of surgical treatment in a cohort of patients with diagnosis of supratentorial spontaneous intracerebral hemorrhage (ICH).
Materials and methodsThe study included all consecutive patients with supratentorial ICH admitted to the Intensive Care Units of three Spanish hospitals with Neurosurgery Department between 2009 and 2012. Data collected: age, APACHE-II, Glasgow Coma Score (GCS), and pupillary anomalies on admission, intracerebral hemorrhage (ICH) score, location/volume of hematoma, intraventricular hemorrhage (IVH), surgical evacuation alone or with additional external ventricular drain, and 30-days survival and at hospital discharge
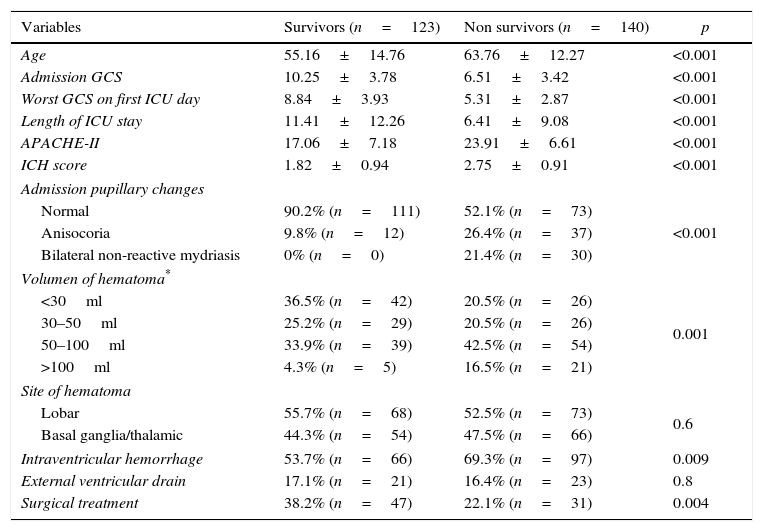
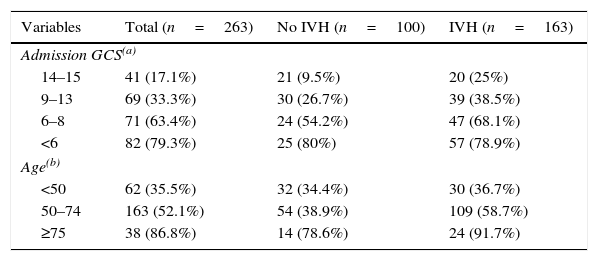
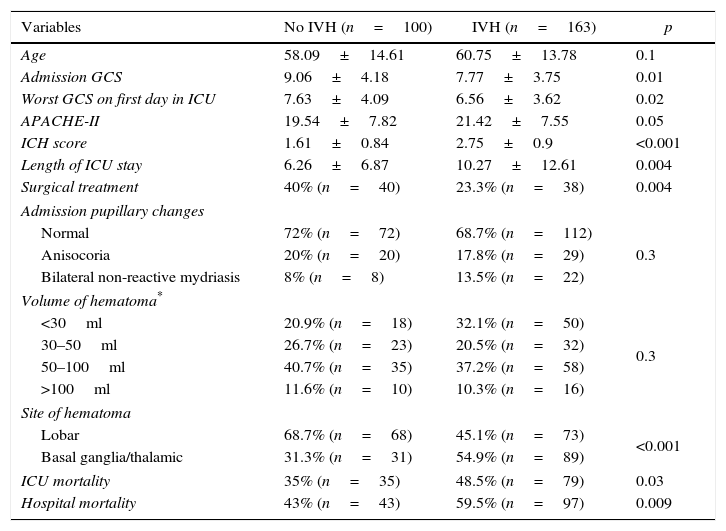
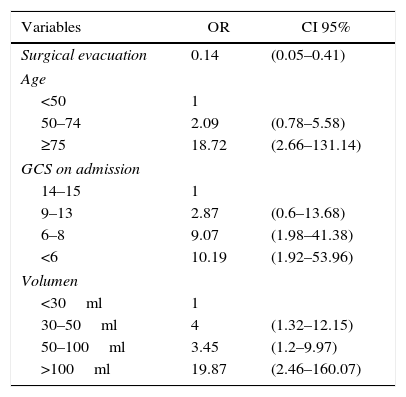
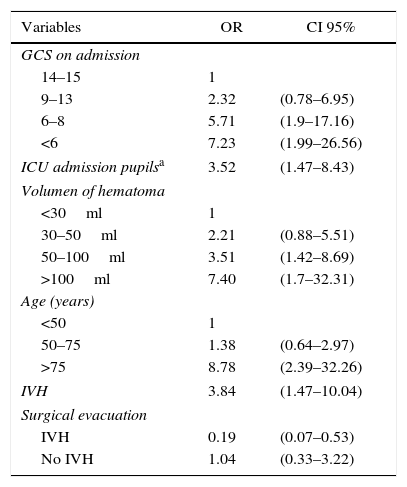
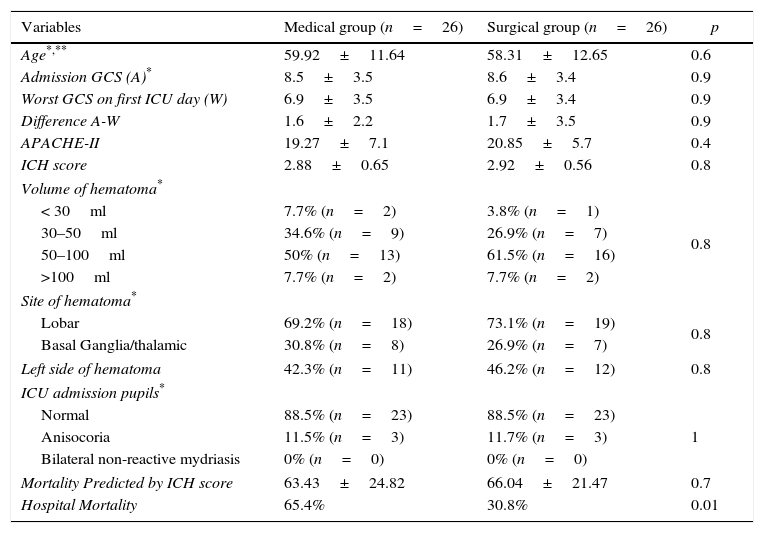
ResultsA total of 263 patients were included. Mean age: 59.74±14.14 years. GCS: 8±4 points, APACHE II: 20.7±7.68 points. ICH Score: 2.32+1.04 points. Pupillary anomalies were observed in 30%. The 30-day mortality: 51.3% (45.3% predicted by ICH-score), and 53.2% at hospital discharge. A significant difference (p=0.004) was observed in hospital mortality rates between surgically treated patients (39.7%, n=78) versus those conservatively managed (58.9%, n=185); specifically in those with IVH surgically treated (34.2%, n=38) versus non-operated IVH (67.2%, n=125), p<0.001. No significant difference was found between mortality rates in patients without IVH. Multiple logistic regression analysis showed an OR for surgery of 1.04 (95% CI; 0.33–3.22) in patients without IVH versus 0.19 (95% CI; 0.07–0.53) in patients with IVH (decreased mortality with surgical treatment). The propensity score analysis for IVH patients showed improved survival of operated group (OR 0.23, 95% CI; 0.07–0.75), p=0.01.
ConclusionsHospital mortality was lower in patients who underwent surgery compared to patients conservatively managed, specifically for the subgroup of patients with intraventricular hemorrhage.
Estudio de supervivencia y evaluación del tratamiento quirúrgico en una cohorte de pacientes con hematoma intracerebral espontáneo supratentorial.
Material y métodosIncluidos todos los pacientes con hematoma cerebral espontáneo supratentorial ingresados en las unidades de cuidados intensivos de 3 hospitales españoles con servicios de neurocirugía (2009-2012). Se recogieron la edad, APACHE-II, escala de coma de Glasgow y alteraciones pupilares al ingreso, intracerebral haemorrhage (ICH) score, localización/volumen del hematoma, presencia de hemorragia intraventricular (IVH), evacuación quirúrgica±drenaje ventricular externo, supervivencia a los 30 días y hospitalaria.
ResultadosDoscientos sesenta y tres pacientes, con edad media 59,74±14,14 años, escala de coma de Glasgow: 8±4 puntos e ICH score: 2,32±1,04 puntos. El 30% presentaba alteraciones pupilares. Mortalidad a los 30 días: 51,3% (predicha por ICH score 45,3%) y hospitalaria 53,2%. Hubo diferencia estadísticamente significativa (p=0,004) entre la mortalidad-hospitalaria de los pacientes intervenidos quirúrgicamente (39,7%; n=78) frente a los tratados de modo conservador (58,9%; n=185), y específicamente para los pacientes intervenidos con IVH (34,2%; n=38) frente a los no operados con IVH (67,2%; n=125), (p<0,001). No hubo diferencias en la mortalidad de los pacientes sin IVH. En el análisis de regresión logística múltiple la OR para la cirugía fue 1,04 (IC 95%: 0,33-3,22) en pacientes sin IVH, frente a 0,19 (IC 95%: 0,07-0,53) en pacientes con IVH. El análisis con índice de propensión para pacientes con IVH demostró mejoría en la supervivencia del grupo operado (OR: 0,23; IC 95%: 0,07-0,75), p=0,01.
ConclusiónLa mortalidad hospitalaria fue menor en los pacientes intervenidos quirúrgicamente en comparación con los tratados de modo conservador, específicamente para el subgrupo de pacientes con IVH.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.