A 24-h-stay in the post-anesthesia care unit (PACU) is a common postoperative procedure after deep brain stimulation surgery (DBS).
ObjectiveWe evaluated the impact of a fast-track (FT) postoperative care protocol.
MethodsAn analysis was performed on all patients who underwent DBS in 2 periods: 2006, overnight monitored care (OMC group), and 2007–2013, FT care (FT group).
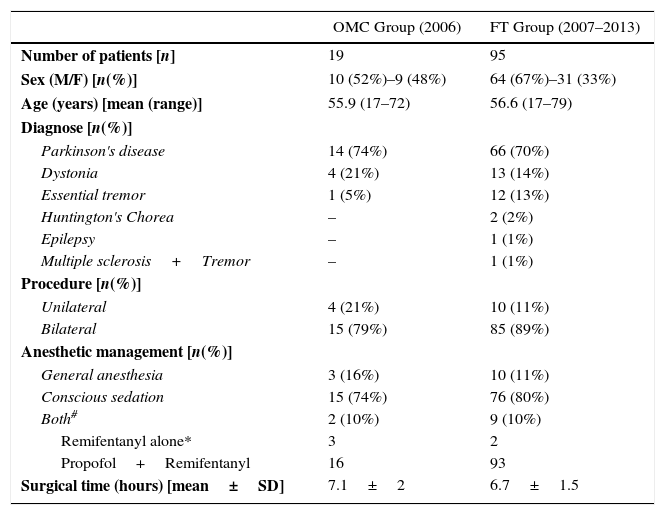
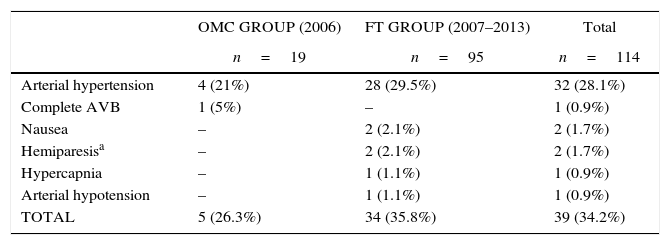
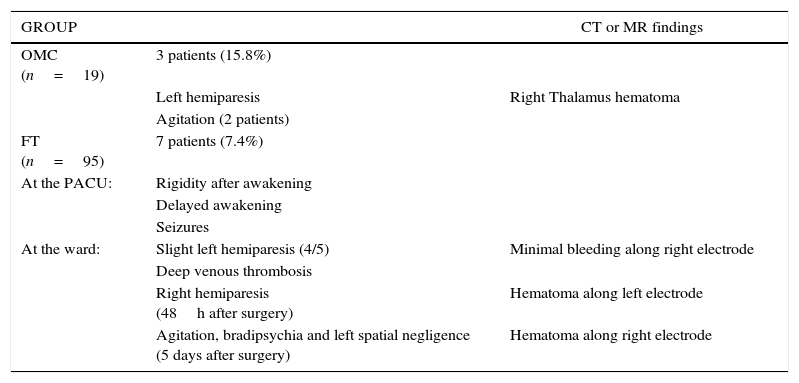
ResultsThe study included 19 patients in OMC and 95 patients in FT. Intraoperative complications occurred in 26.3% patients in OMC vs. 35.8% in FT. Post-operatively, one patient in OMC developed hemiparesis, and agitation in 2 patients. In FT, two patients with intraoperative hemiparesis were transferred to the ICU. While on the ward, 3 patients from the FT developed hemiparesis, two of them 48h after the procedure. Thirty eight percent of FT had an MRI scan, while the remaining 62% and all patients of OMC had a CT-scan performed on their transfer to the ward. One patient in OMC had a subthalamic hematoma. Two patients in FT had a pallidal hematoma, and 3 a bleeding along the electrode.
ConclusionsA FT discharge protocol is a safe postoperative care after DBS. There are a small percentage of complications after DBS, which mainly occur within the first 6h.
La estancia durante 24h en una unidad de recuperación post-anestésica es una estrategia común de control post-operatorio después de la cirugía de estimulación cerebral profunda (DBS).
ObjetivoEvaluamos el impacto de un protocolo Fast-track (FT) en el cuidado postoperatorio.
MétodosAnalizamos todos los pacientes que se sometieron a cirugía DBS en 2 periodos: 2006, monitorización durante la noche (grupo OMC) y entre 2007 y 2013 (grupo FT).
ResultadosIncluimos 19 pacientes en el grupo OMC y 95 pacientes en el FT. Se registraron incidentes intraoperatorios en el 26,3% de pacientes del grupo OMC vs. 35,8% del grupo FT. Postoperatoriamente, un paciente en el grupo OMC desarrollo hemiparesia y 2 pacientes agitación. En el grupo FT, 2 pacientes con hemiparesia intraoperatoria fueron trasladados a la UCI. Durante su ingreso en planta, 3 pacientes del grupo FT desarrollaron hemiparesia, 2 de ellos 48h después del procedimiento. Al 38% del FT se les realizó una resonancia, mientras que al 62% restante y a todos los pacientes del grupo OMC se les realizó un escáner antes del traslado a sala: un paciente del grupo OMC tuvo un hematoma subtalámico; 2 pacientes del grupo FT tuvieron un hematoma en el pálido y 3, sangrado en el trayecto del electrodo.
ConclusionesEl protocolo FT es seguro después de la cirugía de DBS. Hay un pequeño porcentaje de complicaciones y la mayoría suceden en las primeras 6h.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.