To analyze the change in the characteristics of presentation, evolution and treatment in the ICU, as well as the functional evolution at 12 months of spontaneous intracranial hemorrhages (ICHs) treated in an ICU reference center.
Patient and methodsDescriptive, retrospective study in a Neurocritical Reference Hospital. All admissions of patients with HICE during three periods are studied: 1999–2001 (I), 2015–2016 (II) and 2020–2021 (III). Evolution in the three periods of demographic variables, baseline characteristics of the patients, clinical variables and characteristics of bleeding, evolutionary data in the ICU are studied. At one year we assessed the GOS scale (Glasgow Outcome Score) according to whether they had a poor (GOS 1−3) or good (GOS 4−5) prognosis.
Results300 admitted patients, distributed in periods: I: 28.7%, II: 36.3% and III: 35%. 56.7% were males aged 66 (55.5–74) years; ICH score 2 (1−3). The ICU stay was 5 (2–14) days with a mortality of 36.8%. GOS 1−3 a year in 67.3% and GOS 4−5 in 32.7%. Comparing the three periods, we observed a higher prevalence in women, and the presence of cardiovascular factors; no changes in etiology; in relation to the location, it increases cerebellar hemorrhage and in the brainstem. Although the severity was greater, the stay in the ICU, the use of invasive mechanical ventilation and tracheostomy were lower. Open surgery has decreased its use by 50%. Mortality continues to be high, stagnating in the ICU at 35% and entails a high degree of disability one year after assessment.
ConclusionsSevere ICH is a complex pathology that has changed some characteristics in the last two decades, with more severe patients, with more cardiovascular history and a greater predominance of brainstem and cerebellar hemorrhage. Despite the increase in severity, better parameters during the ICU stay, with open surgery used 50% less. Mortality remains stagnant at 35% with high disability per year.
Analizar el cambio de las características de presentación, evolución y tratamiento en UCI, así como la evolución funcional a los 12 meses de las hemorragias intracraneal espontáneas (HICEs) atendidas en una UCI centro de referencia.
Pacientes y métodoEstudio descriptivo, retrospectivo en Hospital de Referencia neurocríticos. Se estudian todos los ingresos de pacientes con HICE durante tres periodos: 1999–2001 (I), 2015–2016 (II) y 2020–2021 (III). Se estudian evolución en los tres periodos de variables demográficas, características basales de los pacientes, variables clínicas y características de la hemorragia, datos evolutivos en UCI. Al año valoramos la escala GOS (Glasgow Outcome Score) según presentaban mal (GOS 1−3) o buen pronóstico (GOS 4−5).
Resultados300 pacientes ingresados, distribuidos en periodos: I: 28,7%, II: 36,3% y III: 35%. 56,7% eran varones con una edad 66 (55,5–74) años; ICH score 2 (1−3). La estancia UCI fue de 5 (2–14) días con una mortalidad del 36,8%. Al año GOS 1−3 en el 67,3% y GOS 4−5 en el 32,7%. Comparando los tres periodos observamos mayor prevalencia en mujeres, y presencia de factores cardiovasculares; no cambios en la etiología; en relación a la localización, aumenta la hemorragia cerebelosa y en troncoencefalo. Aunque la gravedad fue mayor, la estancia en UCI, la utilización de ventilación mecánica invasiva y la traqueostomía son menores. La cirugía abierta ha disminuido su utilización en un 50%. La mortalidad sigue siendo alta, estancada en UCI en el 35% y conlleva un alto grado de discapacidad al año de valoración.
ConclusionesLa HICE grave es una patología compleja que ha modificado algunas características en las dos últimas décadas, con pacientes más graves, con más antecedentes cardiovasculares y mayor predominancia de la hemorragia en tronco-encéfalo y cerebelosa. A pesar de aumento gravedad, mejores parámetros en la estancia en UCI, con una cirugía abierta que se utiliza un 50% menos. La mortalidad sigue estancada en un 35% con una alta discapacidad al año.
Spontaneous intracerebral haemorrhage (SICH) is a prevalent disease. It is the second leading cause of acute cerebrovascular accident (ACVA), accounting for about 10–15% of the total.1,2 The significance of SICH lies in its high rates of morbidity and mortality in a young adult population, with mortality rates ranging from 30% to 50%.3
In addition to neurological complications, these patients often develop multiple systemic complications and, as we also have to consider it a clinical process with different standardised phases of action, specialised multidisciplinary management is required. Management of SICH in the intensive care unit (ICU) and the involvement of the intensive medicine specialist and neurosurgeon in conjunction with other specialists constitute standard practice for this disorder.4 However, the poor outcomes sometimes lead to a sense of futility about the measures applied.5,6
Our objective was to analyse the changes over more than 20 years in the presenting features, outcomes and treatment in the ICU, and in functional prognosis at 12 months after SICH treated in the ICU of a referral hospital.
Patients and methodsThis was a descriptive, retrospective study in a tertiary level hospital, a referral hospital for neurosurgical patients where only multiple trauma and neurocritical patients are treated. Information was collected on all admissions of patients with SICH in three periods: 1999–2001 (period I); 2015–2016 (period II); and 2020–2021 (period III). Demographic variables, baseline patient characteristics, clinical variables and bleeding characteristics, as well as data on progress and outcomes during their stay in the ICU were gathered. The ICH score was used to assess intracerebral haemorrhage, as its components (age, admission Glasgow Coma Scale (GCS), location, volume and extension to the brain's ventricular system) enable easy stratification of patients on admission.7 The different variables were taken from the medical records, paper-based in the first period and computerised in the other two periods. In addition, the Glasgow Outcome Scale (GOS) was assessed at one year.8 For this purpose, the patients' progress was monitored at subsequent follow-up appointments. In patients whose progress was unclear, a telephone call was made to assess their condition. Patients were classified according to the GOS as poor prognosis (GOS 1−3) and good prognosis.4,5
Statistical analysisQuantitative variables are expressed as median (interquartile range [IQR]) and categorical variables as number (percentage). The chi-squared test was used to compare categorical variables. Analysis of variance was used to compare quantitative variables, with post hoc analysis using the Bonferroni test. A p-value <0.05 was considered significant.
Authorisation was obtained from our hospital's Independent Ethics Committee.
ResultsOverall, in our unit, of all patients with multiple trauma and neurocritical injuries over these 23 years, 14.4% were admitted for SICH. This represents 300 admissions, distributed as follows: period I, 86 patients (28.7%); period II, 109 patients (36.3%); and period III 105 patients (35%).
Over the course of the 23 years, 56.7% were male, with a mean age of 66 years (55.5–74). Of all the SICH cases, 42.2% were located in the cerebral lobes, 35.3% in the basal ganglia, 15.2% cerebellar, 4.2% in the brainstem and 3.1% in other locations. There was intraventricular extension in 55%, with 21.3% of patients having placement of an external ventricular drain (EVD). In 60.9% the cause was hypertension, followed by 17.7% in whom it was attributed to taking antiplatelet or anticoagulant drugs. Surgical evacuation was performed in 38.5%. The ICH score was 2 (1−3). Mechanical ventilation was applied in 71.8%, with tracheostomy required in 22.7%. Overall ICU stay was 5 (2–14) days.
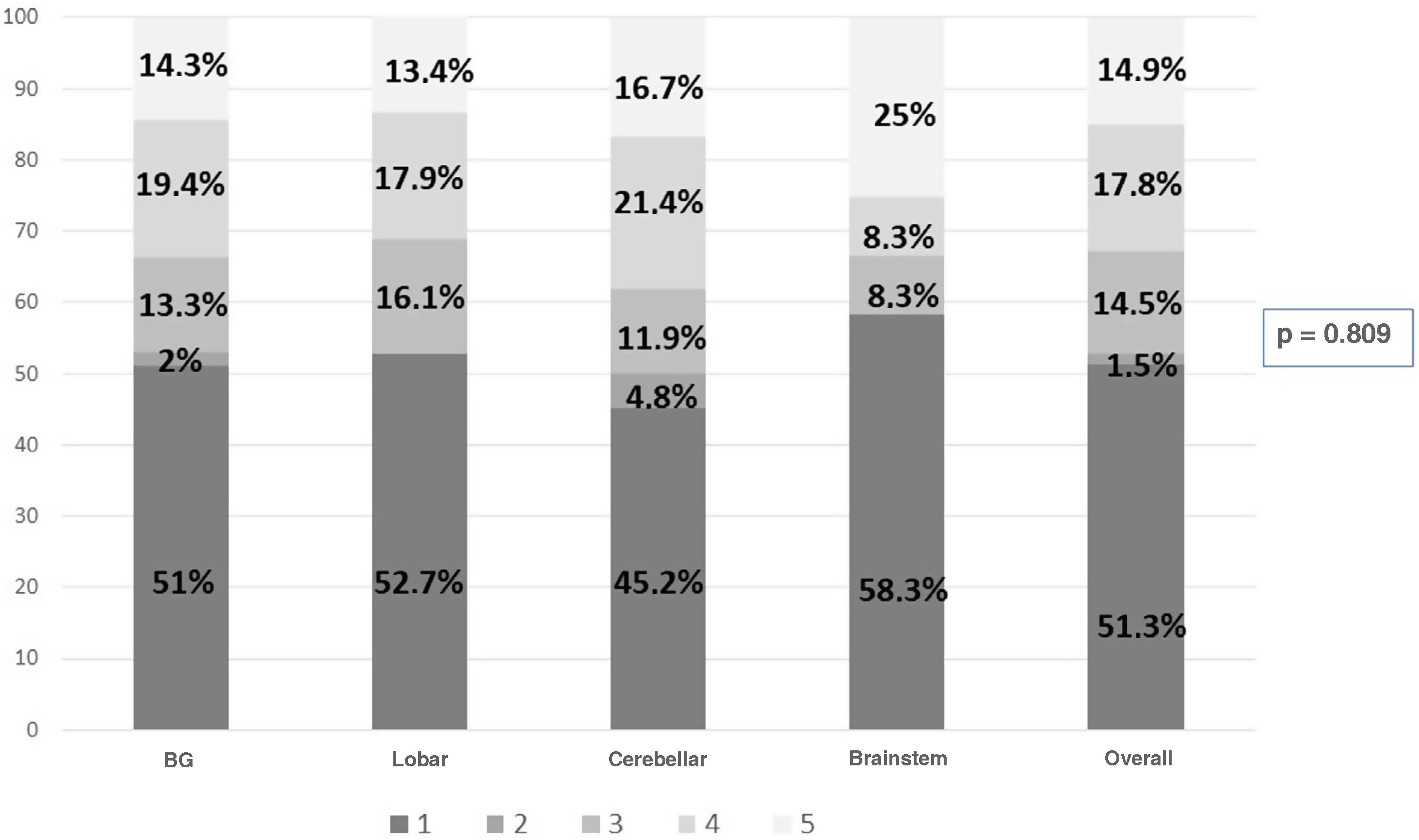
The overall ICU mortality rate was 36.8%. Assessed at one year, the GOS scores were as follows: 1 (death) 51.3%; 2 (persistent vegetative state) 1.5%; 3 (severe disability) 14.5%; 4 (moderate disability) 17.8%; and 5 (minimal disability) 14.9%. The GOS was of poor prognosis in 67.3% and of good prognosis in 32.7%.
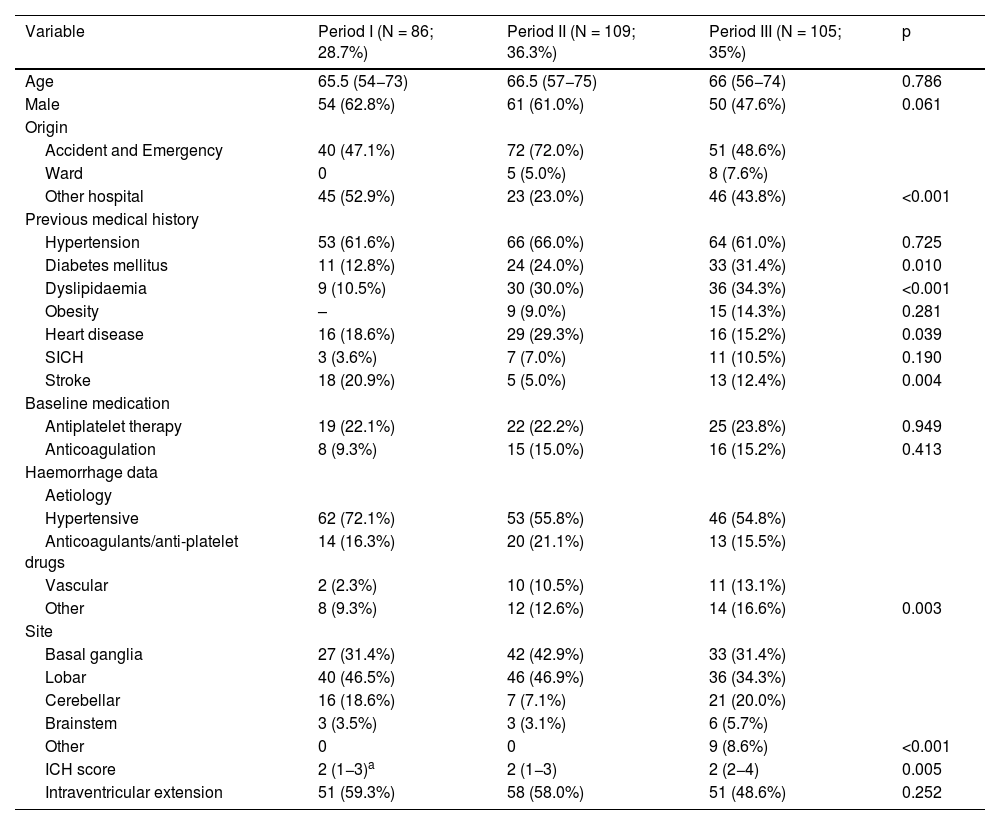
The results in relation to baseline variables, previous medical history and data characteristic of intracranial haemorrhage in the different periods studied are shown in Table 1. GCS on admission, data on ICU progress and outcomes and the assessment of the GOS scale at one year after the episode are shown in Table 2, also for the three periods.
Comparison of baseline variables, previous medical history and haemorrhage data in the three study periods.
| Variable | Period I (N = 86; 28.7%) | Period II (N = 109; 36.3%) | Period III (N = 105; 35%) | p |
|---|---|---|---|---|
| Age | 65.5 (54−73) | 66.5 (57−75) | 66 (56−74) | 0.786 |
| Male | 54 (62.8%) | 61 (61.0%) | 50 (47.6%) | 0.061 |
| Origin | ||||
| Accident and Emergency | 40 (47.1%) | 72 (72.0%) | 51 (48.6%) | |
| Ward | 0 | 5 (5.0%) | 8 (7.6%) | |
| Other hospital | 45 (52.9%) | 23 (23.0%) | 46 (43.8%) | <0.001 |
| Previous medical history | ||||
| Hypertension | 53 (61.6%) | 66 (66.0%) | 64 (61.0%) | 0.725 |
| Diabetes mellitus | 11 (12.8%) | 24 (24.0%) | 33 (31.4%) | 0.010 |
| Dyslipidaemia | 9 (10.5%) | 30 (30.0%) | 36 (34.3%) | <0.001 |
| Obesity | – | 9 (9.0%) | 15 (14.3%) | 0.281 |
| Heart disease | 16 (18.6%) | 29 (29.3%) | 16 (15.2%) | 0.039 |
| SICH | 3 (3.6%) | 7 (7.0%) | 11 (10.5%) | 0.190 |
| Stroke | 18 (20.9%) | 5 (5.0%) | 13 (12.4%) | 0.004 |
| Baseline medication | ||||
| Antiplatelet therapy | 19 (22.1%) | 22 (22.2%) | 25 (23.8%) | 0.949 |
| Anticoagulation | 8 (9.3%) | 15 (15.0%) | 16 (15.2%) | 0.413 |
| Haemorrhage data | ||||
| Aetiology | ||||
| Hypertensive | 62 (72.1%) | 53 (55.8%) | 46 (54.8%) | |
| Anticoagulants/anti-platelet drugs | 14 (16.3%) | 20 (21.1%) | 13 (15.5%) | |
| Vascular | 2 (2.3%) | 10 (10.5%) | 11 (13.1%) | |
| Other | 8 (9.3%) | 12 (12.6%) | 14 (16.6%) | 0.003 |
| Site | ||||
| Basal ganglia | 27 (31.4%) | 42 (42.9%) | 33 (31.4%) | |
| Lobar | 40 (46.5%) | 46 (46.9%) | 36 (34.3%) | |
| Cerebellar | 16 (18.6%) | 7 (7.1%) | 21 (20.0%) | |
| Brainstem | 3 (3.5%) | 3 (3.1%) | 6 (5.7%) | |
| Other | 0 | 0 | 9 (8.6%) | <0.001 |
| ICH score | 2 (1−3)a | 2 (1−3) | 2 (2−4) | 0.005 |
| Intraventricular extension | 51 (59.3%) | 58 (58.0%) | 51 (48.6%) | 0.252 |
ICH: intracerebral haemorrhage; SICH: spontaneous intracranial haemorrhage.
Qualitative variables are expressed as number (percentage) and quantitative variables as median (interquartile range).
Comparison of outcome-related variables during admission to the ICU and at one year in the three study periods.
| Variable | Period I (N = 86; 28.7%) | Period II (N = 109; 36.3%) | Period III (N = 105; 35%) | p |
|---|---|---|---|---|
| Admission data | ||||
| Admission GCS | 9 (7−13) | 12 (6−14) | 10 (5−14) | 0.581 |
| Admission GCS-motor | 6 (4−6) | 6 (4−6) | 5 (2−6) | 0.132 |
| ICU progress data | ||||
| Length of stay | 6 (3−11) | 7 (2−18) | 4 (1.5−11) | 0.028 |
| Invasive mechanical ventilation | 69 (80.2%) | 62 (62.0%) | 78 (74.3%) | 0.018 |
| External ventricular drainage | 19 (22.1%) | 20 (20.0%) | 23 (21.9%) | 0.925 |
| Evacuation surgery | 56 (65.1%) | 23 (23.0%) | 33 (31.4%) | <0.001 |
| Tracheostomy | 22 (25.6%) | 24 (24.0%) | 20 (19.0%) | 0.521 |
| ICU outcomes | ||||
| Ward | 29 (33.7%) | 60 (60.0%) | 65 (61.9%) | |
| Death | 35 (40.7%) | 35 (35.0%) | 37 (35.2%) | |
| Other hospitals | 22 (25.6%) | 5 (5.0%) | 3 (2.9%) | <0.001 |
| Reason for death | ||||
| MODS | 7 (20.0%) | 3 (7.7%) | 6 (16.2%) | |
| LTE | 10 (28.6%) | 12 (30.8%) | 11 (29.7%) | |
| Brain death | 12 (34.3%) | 20 (51.3%) | 14 (37.8%) | |
| Refractory ICHT | 4 (11.4%) | 0 | 4 (10.8%) | |
| Other | 2 (5.7%) | 4 (10.3%) | 2 (5.4%) | 0.371 |
| 1-year outcome data | ||||
| GOS 1−3 | 47 (58.8%) | 67 (72.8%) | 71 (68.9%) | |
| GOS 4−5 | 33 (41.3%) | 25 (27.2%) | 32 (31.1%) | 0.132 |
GCS: Glasgow Coma Scale; GOC: Glasgow Outcome Score; ICHT: intracranial hypertension; ICU: Intensive Care Unit; LTE: Limitation of therapeutic effort; MODS: Multiple Organ Dysfunction Syndrome.
Qualitative variables are expressed as number (percentage) and quantitative variables as median (interquartile range).
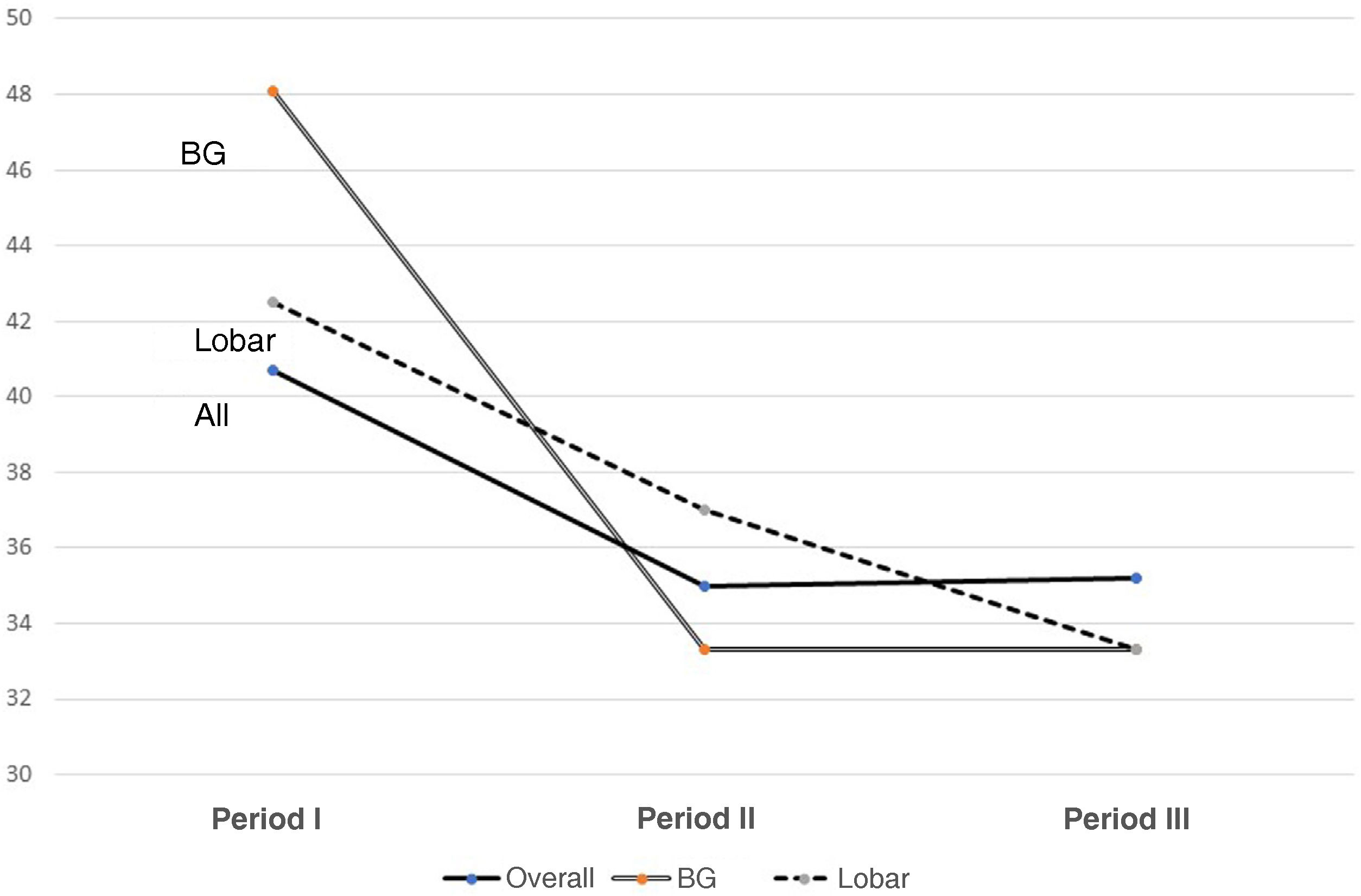
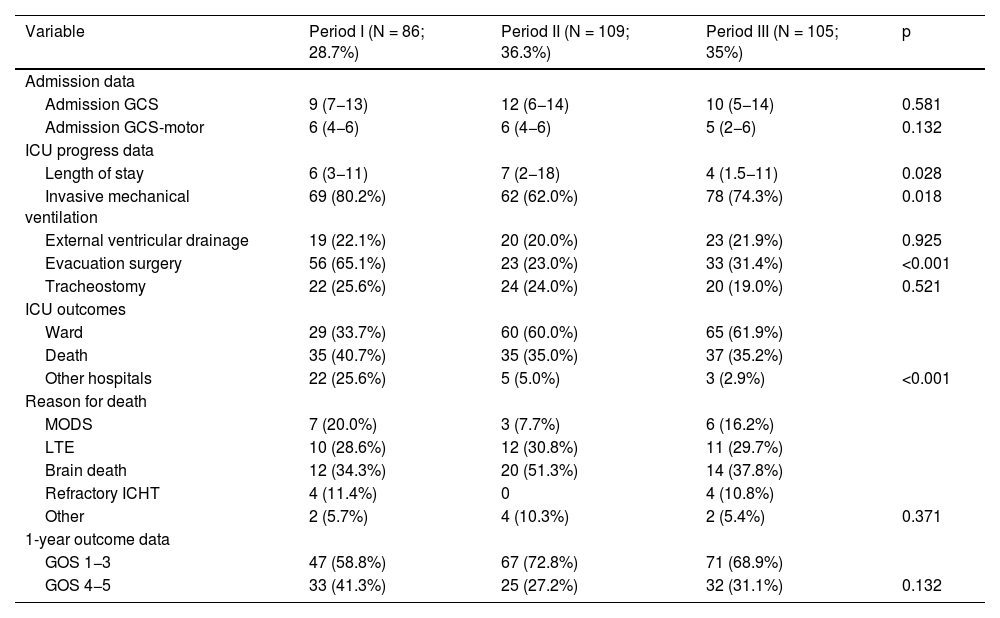
Fig. 1 shows changes in mortality rates over the three periods for the entire group and for basal ganglia and lobar haemorrhages.
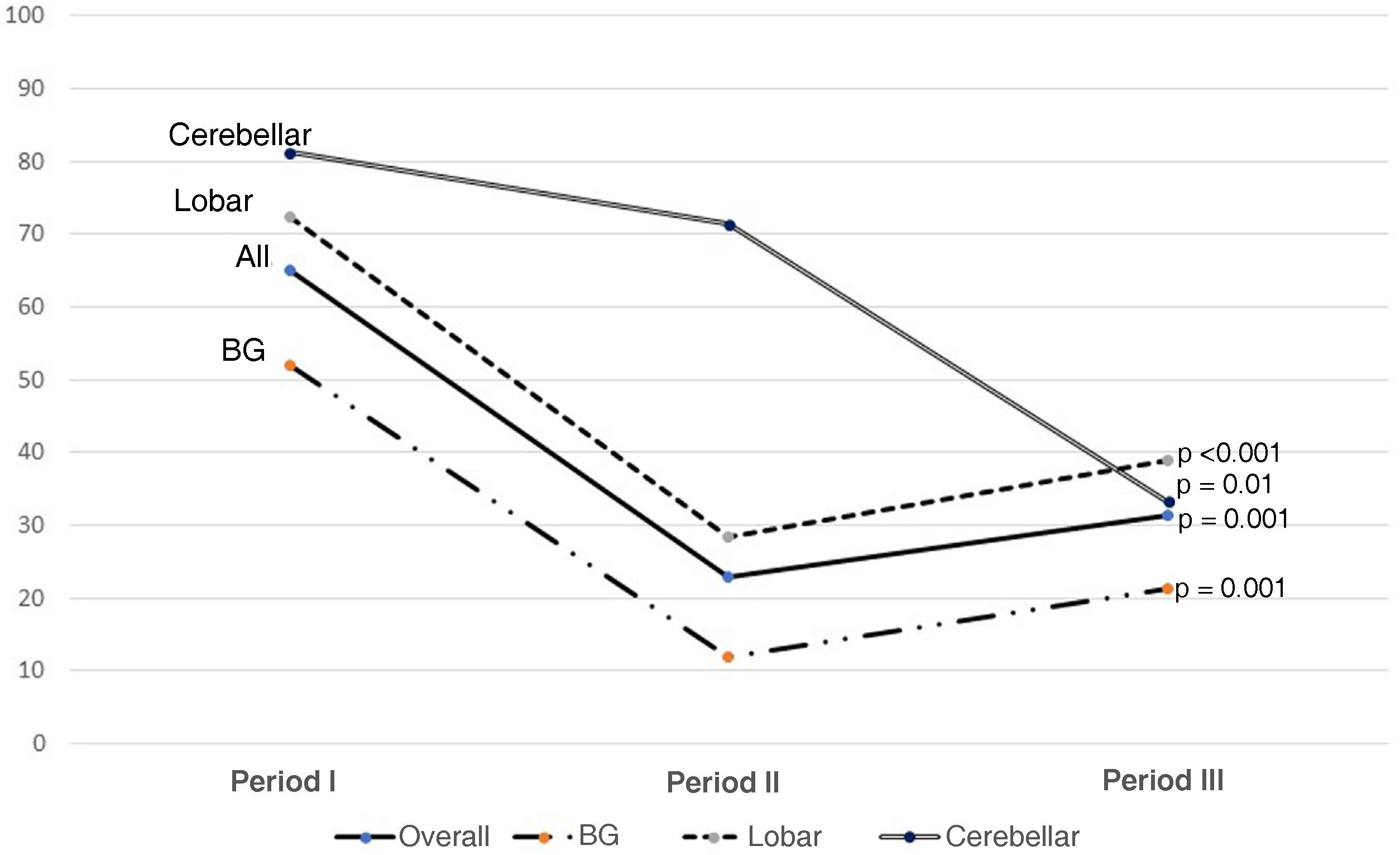
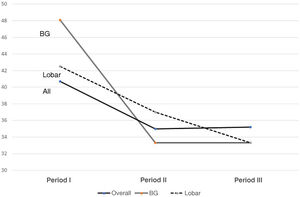
Fig. 2 shows the use of evacuation surgery in the three periods in the total number of patients and according to the type of haemorrhage.
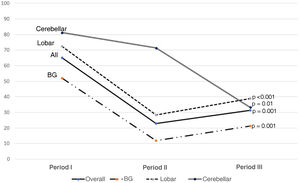
Fig. 3 shows the components of GOS at the one-year assessment in all patients and according to the site of the haemorrhage. It should be noted that lobar and brainstem haemorrhages do not have group 2.
DiscussionThe last twenty or thirty years have seen major advances in neurocritical patient care, accompanied by better initial treatment, better monitoring and more targeted treatment, with the process focused on a better pathophysiological understanding of the factors involved.1,9 This has led to a better specialisation and, over this time at our hospital, our department has evolved from a Multipurpose Intensive Care Medicine Department in period I to a Department of Intensive Care Medicine with a Unit specialising in neurocritical patients (periods II and III). This complexity has led to a large percentage of patients referred from other centres, with the aim of improving the complexity of management and decision-making.
SICH patients account for 10–15% of ACVA.10–12 We found the figures to be in this range in our series, highlighting the significance of this disorder. However, intracranial haemorrhage is not a homogeneous entity, but is determined by the characteristics of the haemorrhage. Although there was no difference in the age of the patients in the periods we studied, we did find a clear trend towards progressively more female SICH patients. Attention has recently been drawn to the under-representation of women in clinical trials on SICH.13
Over the three periods, the prevalence of diabetes, obesity, previous SICH disease and anticoagulated patients progressively increased. This was associated with worse prognosis, with particular emphasis on the use of anticoagulants.14,15 Interesting is the tendency to treat patients who have already had a previous SICH and who have recently been associated with a higher incidence of major cardiovascular events during admission.
Analysing the data related to the haemorrhages, progressively more cerebellar (probably due to the clearer surgical indication) and brainstem haemorrhages have been referred to our Unit, although the patient numbers are very small. In contrast, we have received fewer patients with basal ganglia haemorrhage. The ICH score has increased significantly, indicating greater extent and severity of bleeding. Although early treatment has been associated with a trend towards better survival after controlling for severity (ICH score), it is clear that it is the most severe patients who tend to be referred.1 This is in line with studies showing that patients who are not comatose or who do not need invasive mechanical ventilation in the first 24 h can be monitored and treated outside the ICU or in non-specialised ICUs.16,17
In relation to the ICU data, we found a significant decrease in the use of invasive mechanical ventilation, tracheostomy and length of stay in the different periods. Although we are unable to draw firm conclusions from this, the progressive improvement in the management of such patients in referral centres and in mechanical ventilation has been shown to impact on patient care.18,19
Surgical indications have been one of the cornerstones of treatment. In our series, we found a very significant decrease in the number of patients requiring surgery. This decreasing role of surgery, as can be seen in Fig. 2, is in line with the findings of the last 20 years. Trials of the size of the Surgical Trial in Intracerebral Haemorrhage (STICH I), Surgical Trial in Lobar Intracerebral Haemorrhage (STICH II), Minimally Invasive Surgery plus rt-PA for Intracerebral Haemorrhage Evacuation (MISTIE) or Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Haemorrhage Phase III (CLEAR III) have not demonstrated statistically significant mid- and long-term functional improvement, although the tendency is to believe that they reduce the fatality of neurological outcome20–22; this suggests that the ideal surgical management is yet to be determined.11,23 Not surprisingly, at a theoretical level, drainage of the haematoma has many benefits, making it possible to reduce the mass effect and consequent brain herniation, with the secondary damage caused by excitotoxicity and neurotoxicity of the blood products.24
Despite a lack of certainty about the benefits of neurosurgery, there is sufficient evidence to support certain surgical interventions, such as the placement of external ventricular drains, both for monitoring and for control of hydrocephalus related to bleeding into the ventricles; 51%–58% of patients in our series had intraventricular haemorrhage, which in itself is an independent factor of poor prognosis. Ventricular catheter implantation also allows the instillation of fibrinolytics to facilitate clot dissolution.11
With regard to craniotomies, given the lack of significant results in the aforementioned studies (STICH I or II), we noted a decrease in indications, based on a clear differentiation between supra- and infratentorial SICH. In infratentorial SICH, external ventricular drain (EVD) placement combined with posterior fossa craniotomy and evacuation has been shown to be effective therapy, especially in cerebellar haemorrhages larger than 3 cm in size, with early surgery indicated. In supratentorial SICH, we tend to individualise cases with regard to the indication for surgical evacuation, with age, comorbidities, non-dominance, mass effect and distance from the cortex being factors taken into account before operating.1,11Fig. 2 shows that surgery at all sites has fallen (almost half), with the cerebellar sites showing the smallest decrease, as the above-mentioned factors such as hydrocephalus are taken into consideration.
The role of decompressive craniectomy associated with evacuation is minimal, linked to cases of poor prognosis and poor clinical status (GCS ≤ 8). Although this intervention can safely prevent intracranial hypertension not controllable with pharmacological measures, with good clinical outcomes, it is frequently associated with complications related to the surgical wound and outcomes can be frustrated by the severity of the patients selected.25 One issue pending at our hospital is to address the minimally invasive techniques that are starting to gain ground in large neurosurgical centres worldwide, with the use of endoscopic or neuronavigation techniques, such as the Stereotactic ICH Underwater Blood Aspiration (SCUBA) system, which aim to minimise the morbidity associated with surgery, with satisfactory evacuation rates.1 The progressive implementation of less invasive techniques may open up new avenues of exploration.26
The mortality rates improved only slightly, but not significantly, from Period I to II, with one third of patients continuing to die in the ICU.22 Although this is within the range reported in the literature, many authors draw attention to the fact that the early mortality rate has remained unchanged at 30–40% for more than 20 years.27,28 It is striking that the one-year mortality rate is 51.3%, indicating the seriousness of the disorder.
This devastating disorder not only has an impact in terms of patient deaths, but also in terms of functional status in survivors leading to significant changes in quality of life. The use of rehabilitation teams helps to improve not only functional prognosis, but also post-discharge mortality rates.29 Assessed at one year, 32.7% of our patients showed adequate functional recovery to enable them to lead a reasonably independent life (GOS 4−5), which is in line with the published range of 30–40%.30Table 2 shows that cerebellar haemorrhages achieve a GOS score of 4−5 in 38.1% of patients at one year post-event.
Our study has several limitations, not only in the retrospective review of cases, but setting the study periods in an arbitrary way could certainly influence the results, although we have tried to look at trends rather than absolute cut-off points. Moreover, the periods are not homogeneous, as the first period covered three years and the others two years, but we wanted to increase the size of comparison of the number of patients between the periods. In addition, there are variables with relevant prognostic factors that we could have considered, such as evaluation of the haemorrhage volume in each location, midline shift or the state of the basal cisterns, and even establishing different analyses according to the various distinct aetiologies (for example, coagulopathy, vascular). However, our aim was to provide an overview of a disorder that has continued to be important over the years, without entering into considerations that would need to be made on an individual basis.
In conclusion, severe SICH requires a complex approach and management in specialised units. Over the last twenty or so years, some presenting features in the ICU have changed, with a higher prevalence in women and a greater likelihood of cardiovascular factors; although the aetiology has not changed, the severity has increased and, in terms of location, we have seen an increase in cerebellar and brainstem haemorrhage. While the degree of severity has been greater, length of ICU stay and the use of invasive mechanical ventilation and tracheostomy have decreased. The use of open surgery has decreased by 50%. The mortality rate remains high, stalling at 35% in the ICU, and the degree of disability at the one-year assessment is high.
FundingOur work received no funding from public, private, research or foundation bodies or any other source.
Conflicts of interestThe authors declare that they have no conflicts of interest.