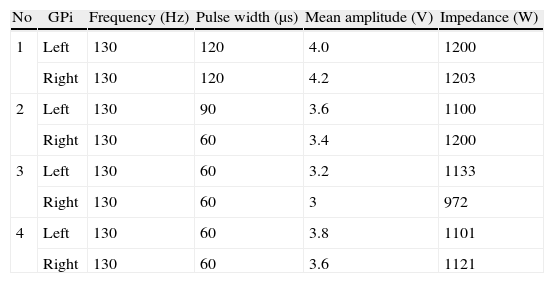
Deep brain stimulation (DBS) of the globus pallidus internus (GPi) is a promising therapeutic option for patients with medically refractory dystonia. We present the results after 1 year of DBS of the GPi in 4 patients with cervical dystonia.
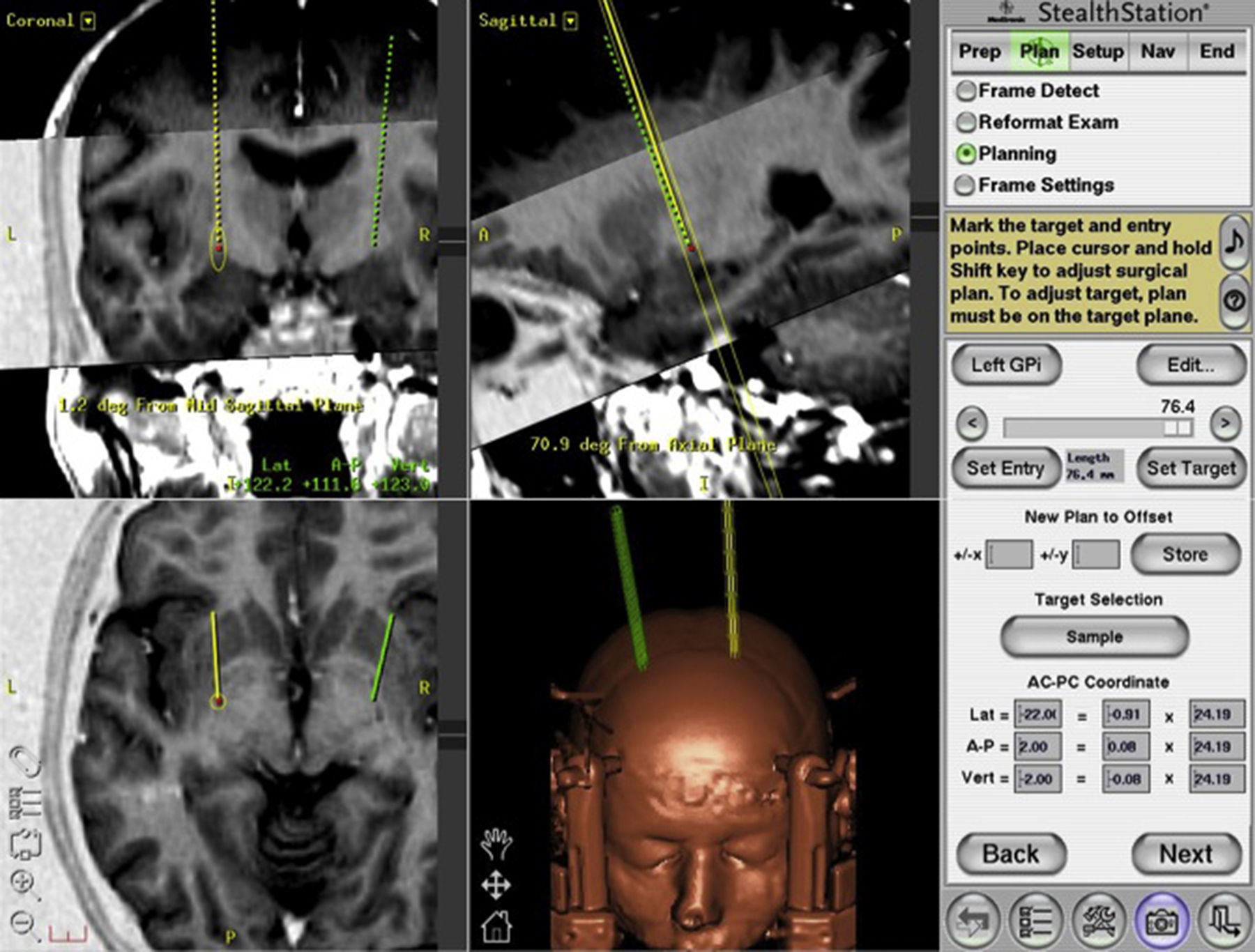
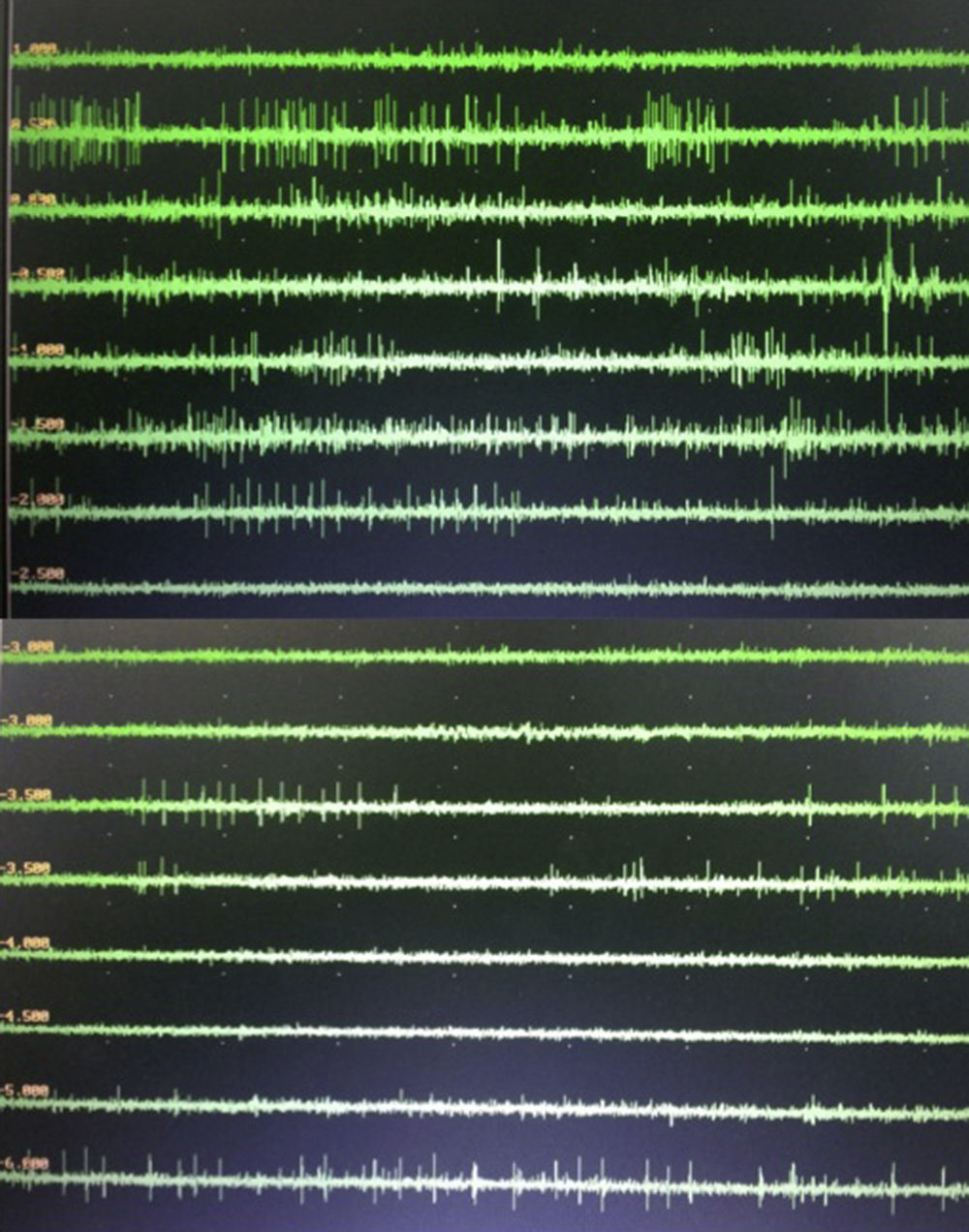
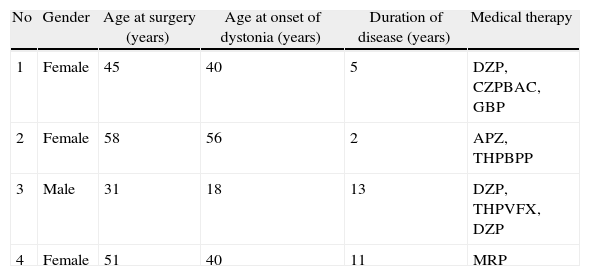
Materials and methodsFour patients with medically refractory cervical dystonia who underwent stereotactic pallidal DBS surgery between June 2010 and November 2011 were included in this retrospective study. Preoperative and postoperative evaluations at 3, 6 and 12 months after surgery were performed using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS).
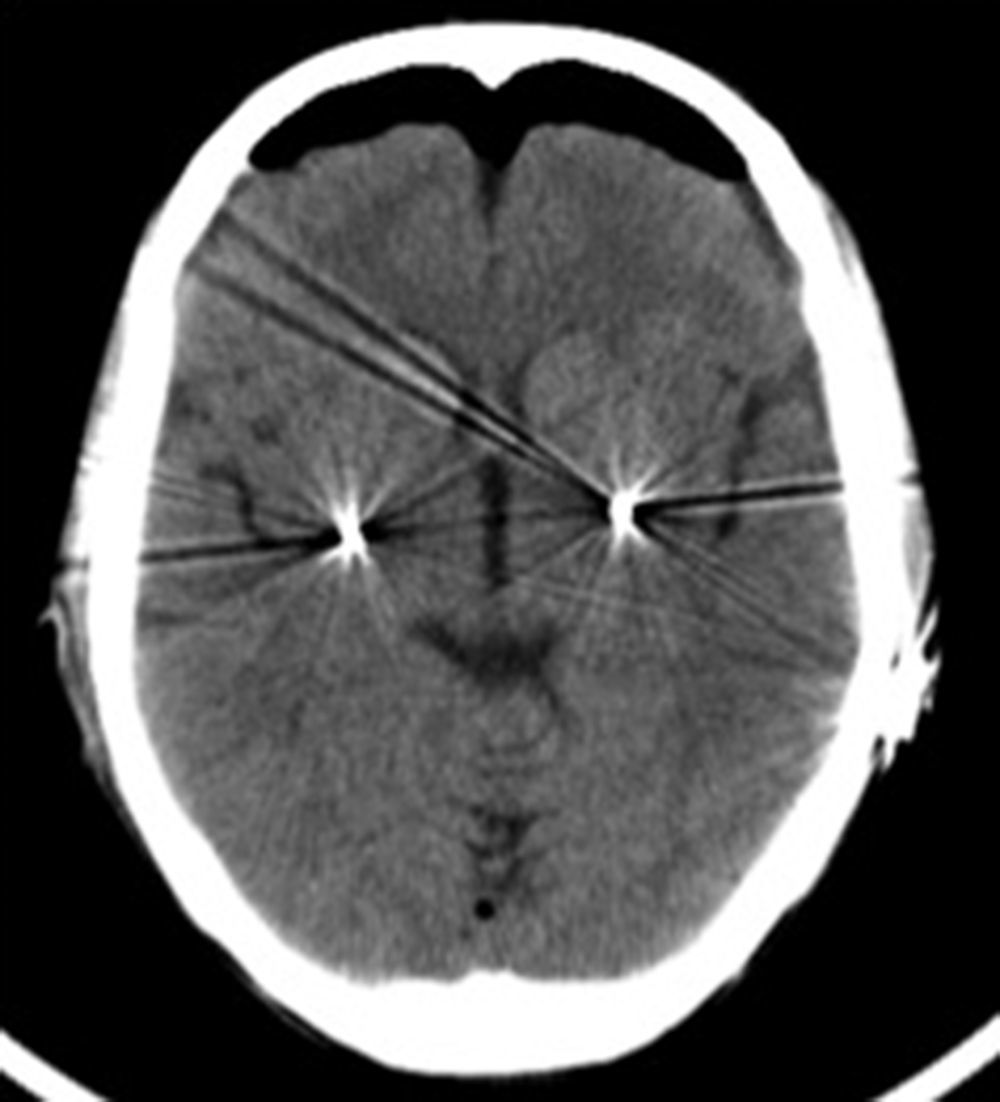
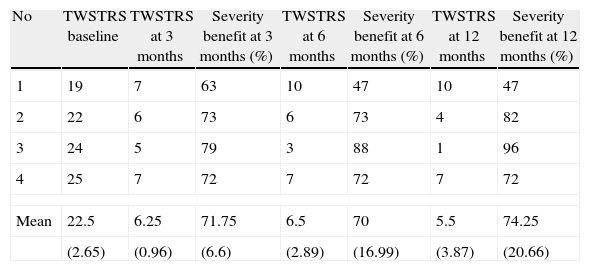
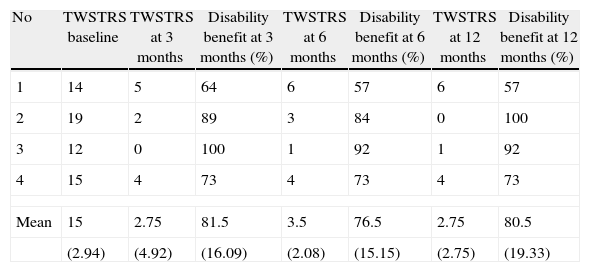
ResultsThe 4 patients experienced a sustained improvement, with a mean TWSTRS reduction of 74.25%, at 12 months follow-up. Disability improved by 80.5% (mean) at 1 year follow-up. No stimulation-related side effects were reported.
ConclusionPallidal DBS is a valid and effective second-line treatment for patients with cervical focal dystonia. Our results support its use in patients with an insufficient response to medical treatment.
La estimulación cerebral profunda (ECP) del globo pálido interno (GPi) es una terapéutica eficaz para pacientes con distonía refractaria al tratamiento médico. Presentamos 4 pacientes con distonía cervical refractaria operados con ECP utilizando como diana el GPi y los resultados un año después de la cirugía.
Material y métodosFueron incluidos en este estudio retrospectivo 4 pacientes con distonía cervical refractaria al tratamiento médico que fueron sometidos a cirugía estereotáctica para ECP del GPi entre junio 2010 y noviembre 2011. Fueron realizadas evaluaciones pre y postoperatorias a los 3, 6 y 12 meses utilizando la escala Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS).
ResultadosEn los 4 pacientes se verificó un beneficio sustentado con una reducción media del 74,25% en las TWSTRS a los 12 meses de seguimiento. Un año después de la cirugía se obtuvo una mejoría media en la discapacidad del 80,5%. En ninguno de los pacientes de encontraron efectos secundarios relacionados con el procedimiento.
ConclusionesECP del GPi es un tratamiento quirúrgico válido y eficaz para la distonía cervical focal. Nuestros resultados apoyan su uso en aquellos pacientes con respuesta insuficiente al tratamiento médico.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.