Cranioplasty is carried out for cosmetic reasons and for protection, but it may also lead to some neurological improvement after the bone flap placement. Complications of cranioplasty are more frequent than expected for a scheduled neurosurgical procedure. We tried to identify factors associated with both complications and improvement after cranioplasty.
MethodsWe prospectively studied the cranioplasties performed in our hospital from November 2009 to November 2013. Patients whose initial reason for bone removal was tumor infiltration were excluded. Demographic, clinical and radiological data were collected. The NIH Stroke Scale and Barthel Self-Care Index scores were obtained both before and within 72h after cranioplasty. The outcome measures were the occurrences of complications and clinical improvement.
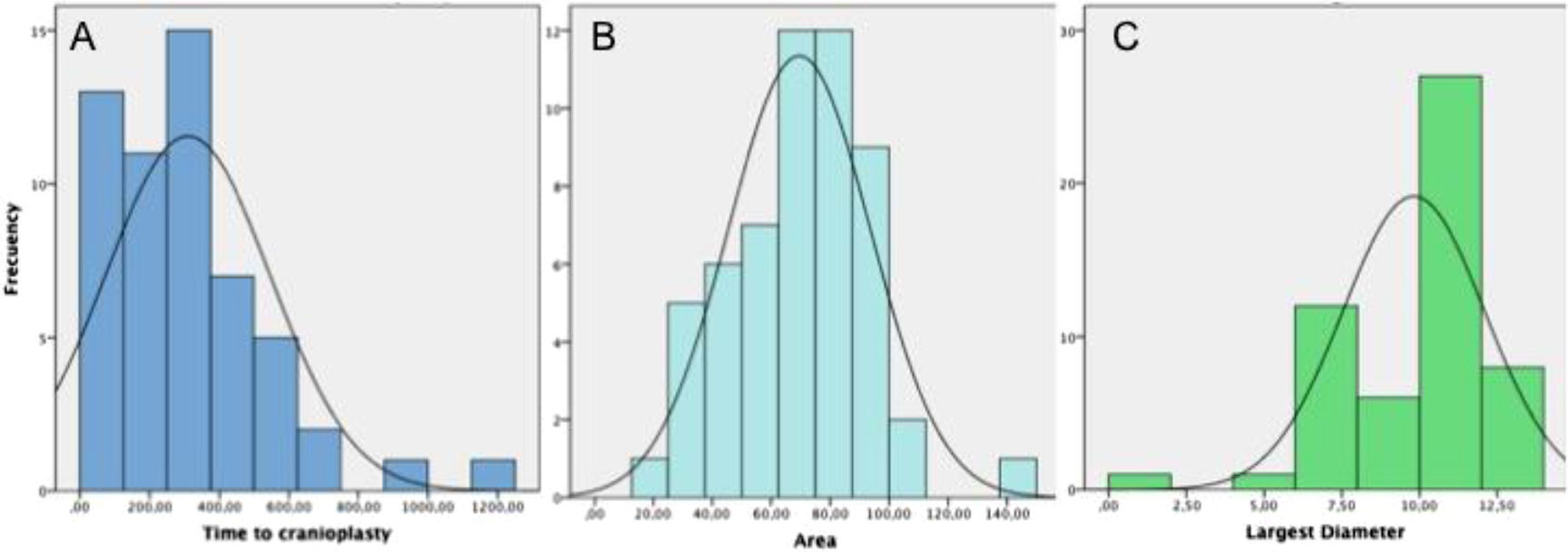
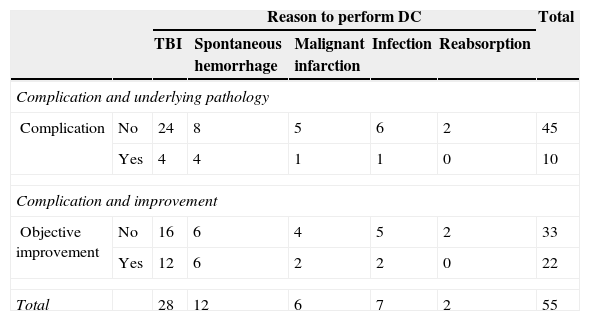
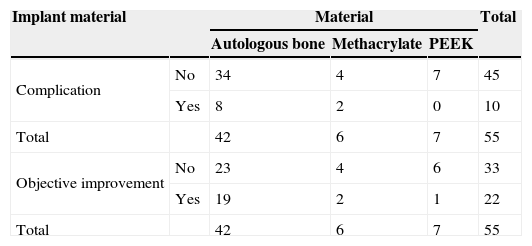
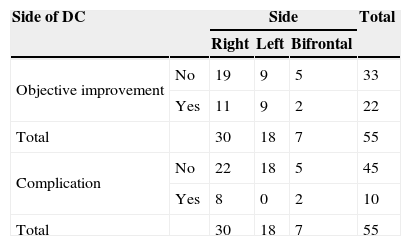
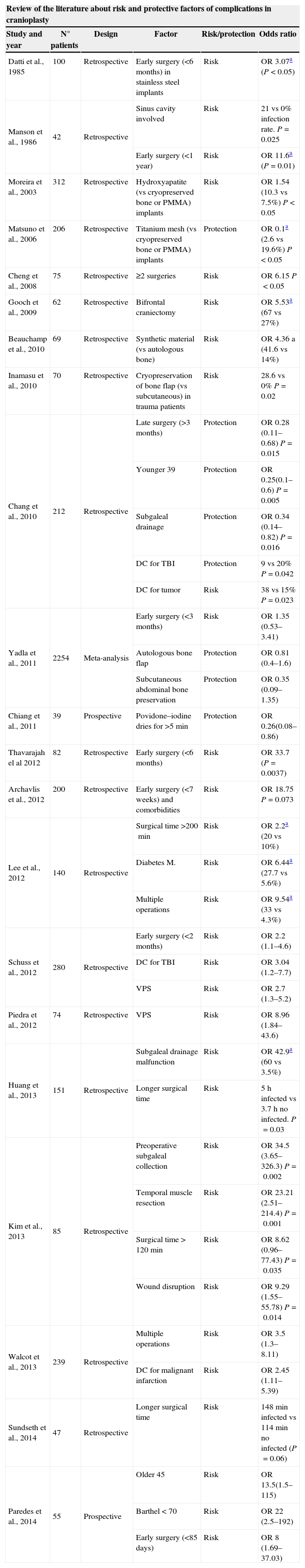
ResultsFifty-five cranioplasties were performed. The material used for the cranioplasty was autologous bone in 42 cases, polyetheretherketone (PEEK) in 7 and methacrylate in 6. The average size of the bone defect was 69.5 (19.5–149.5)cm2. The time elapsed between decompressive craniectomy and cranioplasty was 309 (25–1217) days. There were 10 complications (7 severe and 3 mild), an 18.2% complication rate. Statistically significant risk factors of complications were identified as a Barthel ≤70 (Odds ratio [OR] 22; 2.5–192; P=0.005), age over 45 years (OR 13.5; 1.5–115; P=0.01) and early surgery (≤85 days; OR 8; 1.69–37.03, P=0.004). After multivariate analysis, Barthel ≤70 and age over 45 years remained independent predictors of complications. Twenty-two (40%) of the 55 patients showed objective improvement. Early surgery (<85 days) increased the likelihood of improvement (OR 4.67; 1.05–20.83; P=0.035). Larger bone defects seemed to be related with improvement, but differences in defect size were not statistically significant (75.3 vs 65.6cm2; P=0.1).
ConclusionsThe complication rate of cranioplasty is higher than for other elective neurosurgical procedures. Older age, poorer functional situation (worse Barthel index score) and early surgery (≤85 days) are independent risk factors for complications. However, cranioplasty produces clinical benefits beyond protection and esthetic improvement. Earlier surgery and larger bone defects seem to increase the likelihood of clinical improvement.
La craneoplastia es un procedimiento que se realiza por motivos estéticos y de protección, pero que además puede producir cierta mejoría neurológica tras la reposición del colgajo óseo. Las complicaciones del procedimiento son más frecuentes de lo esperado para un procedimiento neuroquirúrgico programado. Se han tratado de identificar los factores asociados tanto a la aparición de complicaciones como de mejoría neurológica.
MétodosSe han analizado prospectivamente las craneoplastias realizadas en nuestro centro desde noviembre del 2009 hasta noviembre del 2013. Los pacientes sometidos a craniectomía descompresiva (CD) por infiltración tumoral no fueron incluidos. Se recogieron datos demográficos, clínicos y radiológicos. La escala NIHSS y Barthel fueron medidas en cada paciente antes y dentro de las primeras 72h tras la cirugía. Las medidas «resultado» fueron la aparición de complicación y/o mejoría clínica.
ResultadosSe realizaron 55 craneoplastias. El material utilizado para las plastias fue el propio hueso en 42 casos, PEEK en 7 y metacrilato en 6. El tamaño medio del defecto óseo fue de 69,5 (19,5–149,5)cm2. El tiempo medio transcurrido desde la CD hasta la plastia fue de 309 (25–1.217) días. Hubo 10 complicaciones (7 graves, 3 leves), lo que supone una tasa de complicaciones del 18,2%. Una puntuación de Barthel ≤70 (OR: 22; 2,5–192; P=0,005), la edad por encima de 45años (OR: 13,5; 1,5–115; P=0,01), y la cirugía temprana (≤85días, OR: 8; 1,69–37,03, P=0,004) fueron identificados como factores de riesgo estadísticamente significativos de la aparición de complicaciones. Tras el análisis multivariante, el Barthel ≤70 y la edad mayor de 45años permanecieron como predictores independientes de complicaciones. Veintidós (40%) de 55 pacientes presentaron mejoría clínica objetiva. La cirugía temprana (<85 días) aumentó la probabilidad de mejoría (OR: 4,67; 1,05–20,83; P=0,035). El mayor tamaño de defecto óseo parece relacionarse con la aparición de mejoría, pero las diferencias en tamaño entre los que mejoraron y los que no, no resultó estadísticamente significativa (75,3 vs 65,6cm2, P=0,1).
ConclusionesLa tasa de complicaciones de la craneoplastia es mayor que la de otros procedimientos neuroquirúrgicos electivos. Una edad mayor, una peor situación funcional (entendido como peor puntuación en la escala Barthel) y la cirugía temprana (menos de 85días) son factores de riesgo de complicación. Por otro lado, la craneoplastia produce un beneficio clínico más allá de la protección y la mejoría estética. La cirugía temprana y los defectos óseos mayores parecen aumentar la probabilidad de mejoría clínica.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.