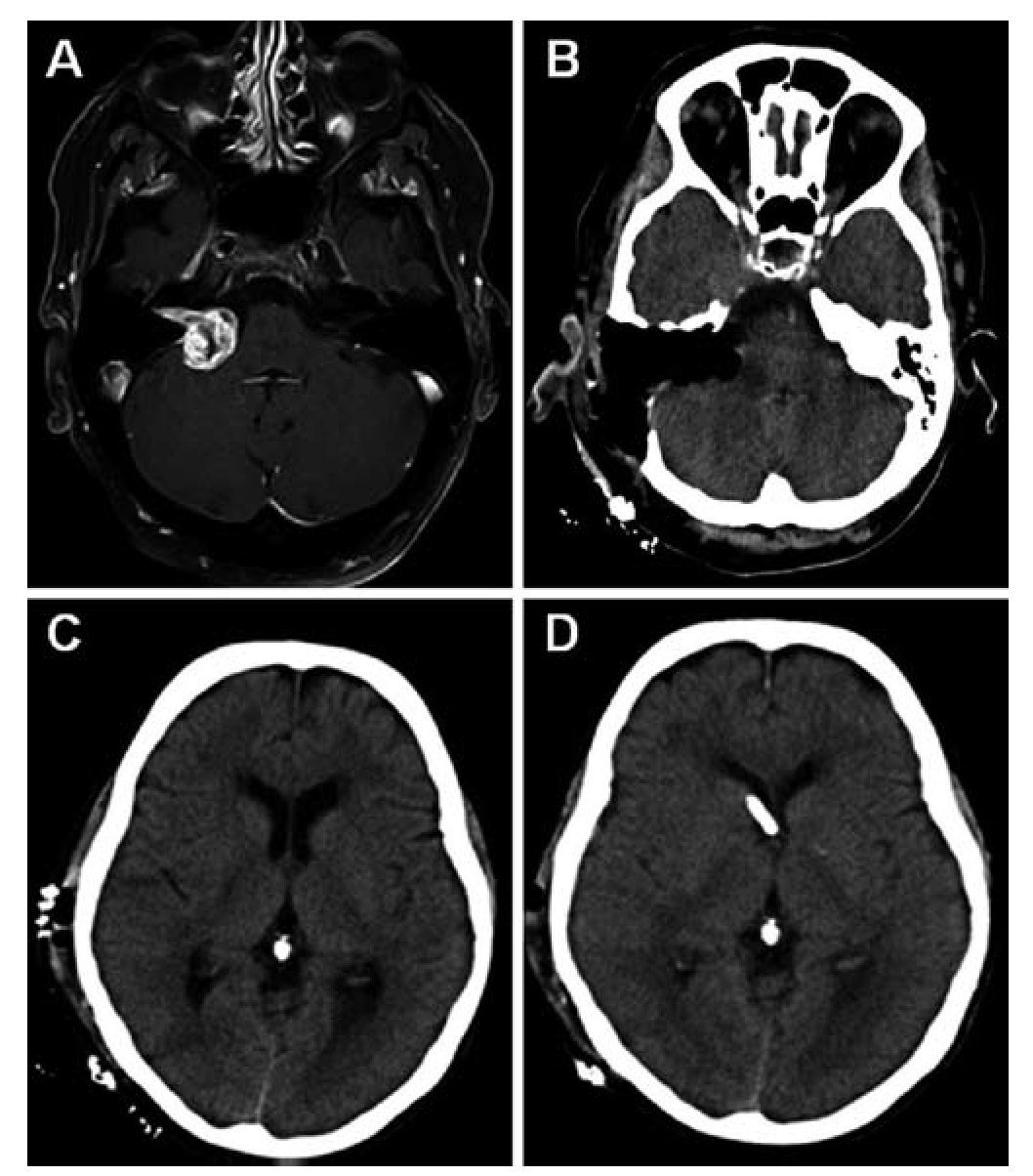
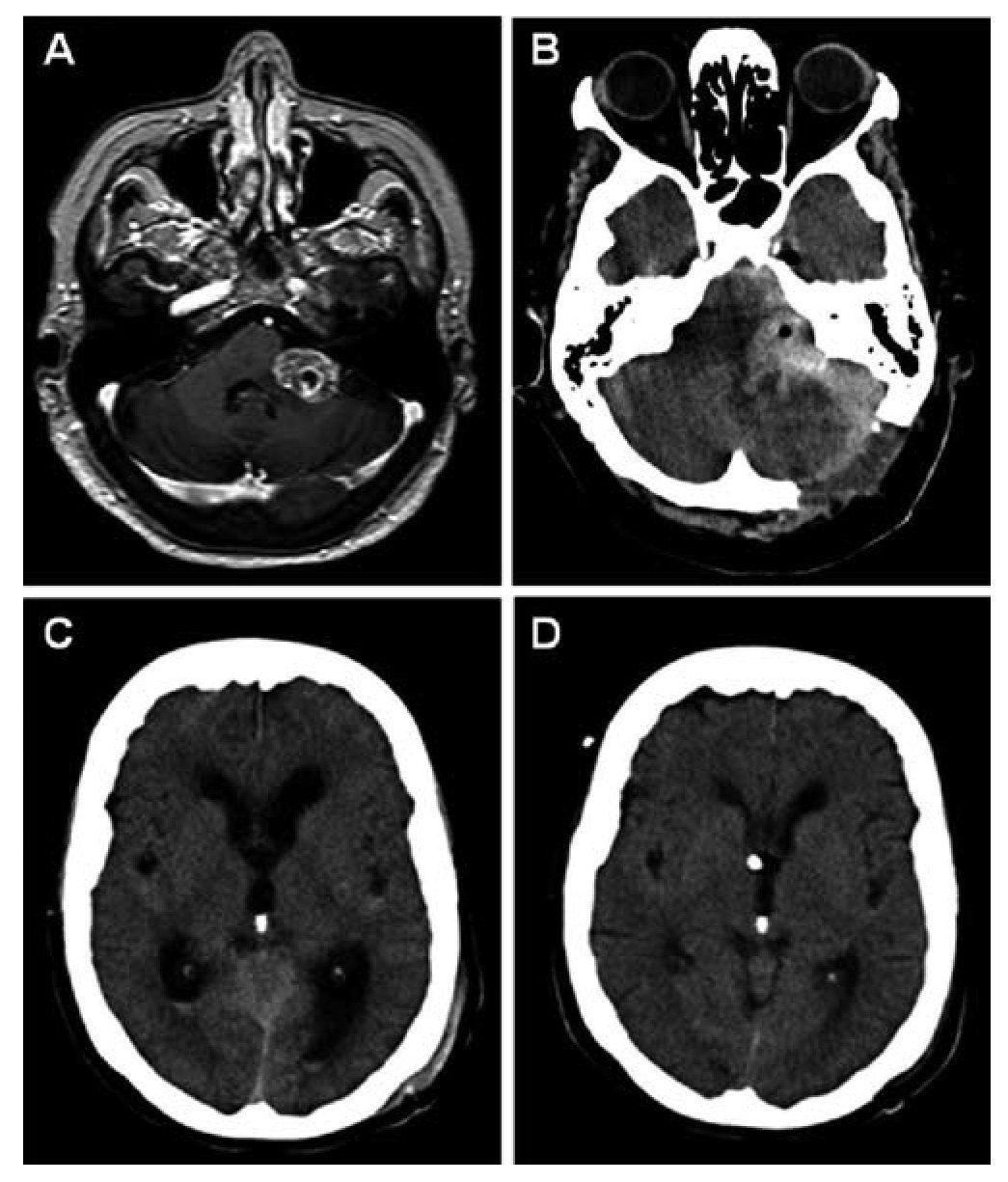
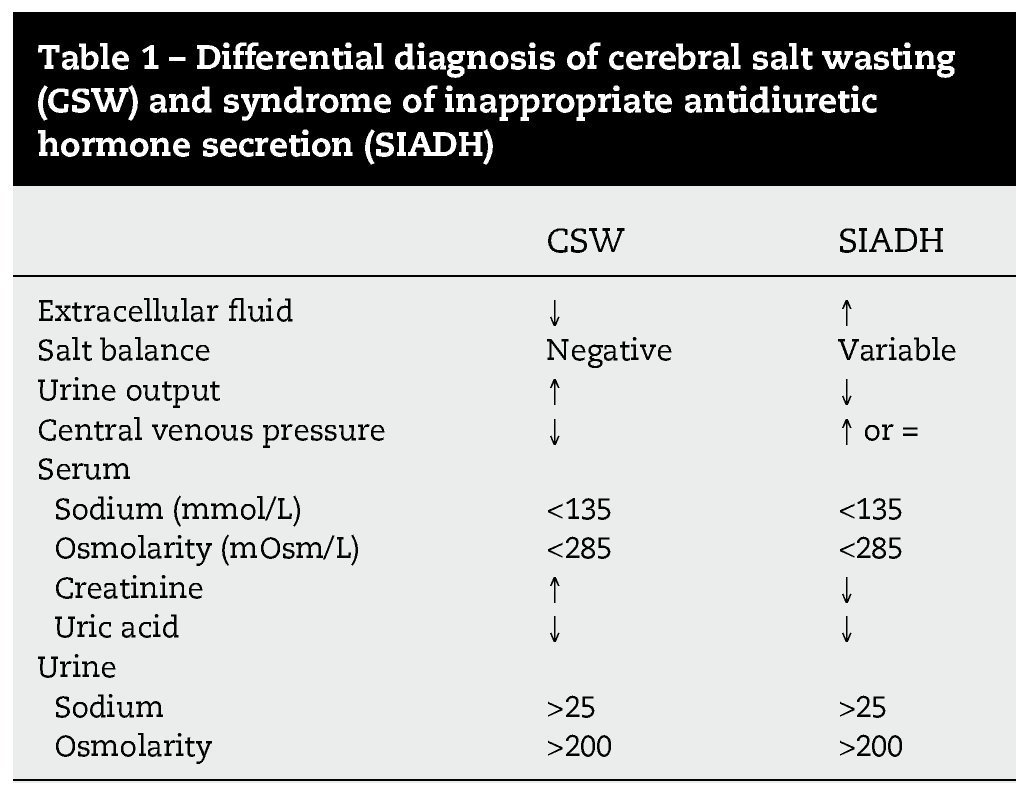
El síndrome pierde sal cerebral (CSW, en sus siglas en inglés) es una complicación rara en la cirugía de los tumores de la fosa posterior. Presentamos a 2 pacientes con tumores del ángulo pontocerebeloso que desarrollaron un CSW posquirúrgico. Ambos pacientes tuvieron un empeoramiento pese a la fluidoterapia y la reposición de sal intensivas. La tomografía computarizada (TC) mostraba una dilatación ventricular leve a moderada que fue tratada mediante un drenaje ventricular externo. Tras la resolución de la hidrocefalia el balance hidroelectrolítico se normalizó rápidamente en ambos pacientes y su situación clínica mejoró. La identificación y el tratamiento precoz de la hidrocefalia obstructiva pueden contribuir al tratamiento del síndrome pierde sal asociado a la cirugía de tumores de la fosa posterior.
Cerebral salt wasting (CSW) is a rare complication in posterior fossa tumour surgery. We present two patients with cerebellopontine angle (CPA) tumours who developed cerebral salt wasting postoperatively. Both patients deteriorated in spite of intensive fluid and salt replacement. On CT scan the patients presented mild to moderate ventricular dilation, which was treated with an external ventricular drainage. After the resolution of hydrocephalus, fluid balance rapidly returned to normal in both patients and the clinical status improved. Identification and treatment of secondary obstructive hydrocephalus may contribute to the management of CSW associated to posterior fossa tumour surgery.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.