Introducción: Aunque la mayoría de los accidentes cerebrovasculares isquémicos son de origen tromboembólico y su tratamiento son endovasculares o médico, algunos son de origen hemodinámico y su manejo puede ser quirúrgico. Hemos revisamos las indicaciones del bypass en el infarto isquémico, la selección de pacientes y las técnicas quirúrgicas utilizadas en nuestra práctica actual.
Métodos: Bypasses extra-intracraneales (EC-IC) de la arteria temporal superficial a arteria cerebral media (STA-MCA), bypasses de alto flujo con interposición de injertos y técnicas de reconstrucción se utilizaron para el tratamiento de pacientes con isquemia sintomática.
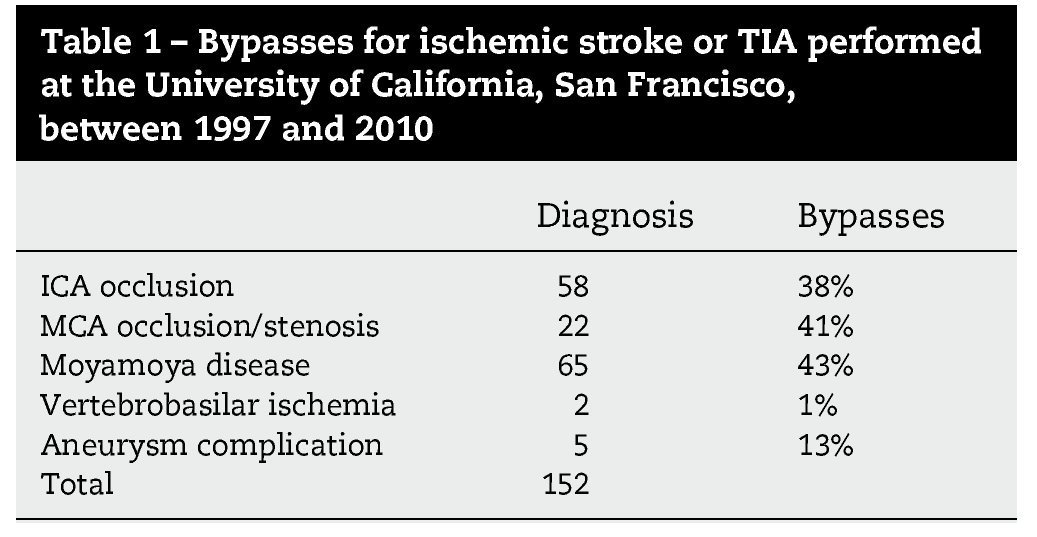
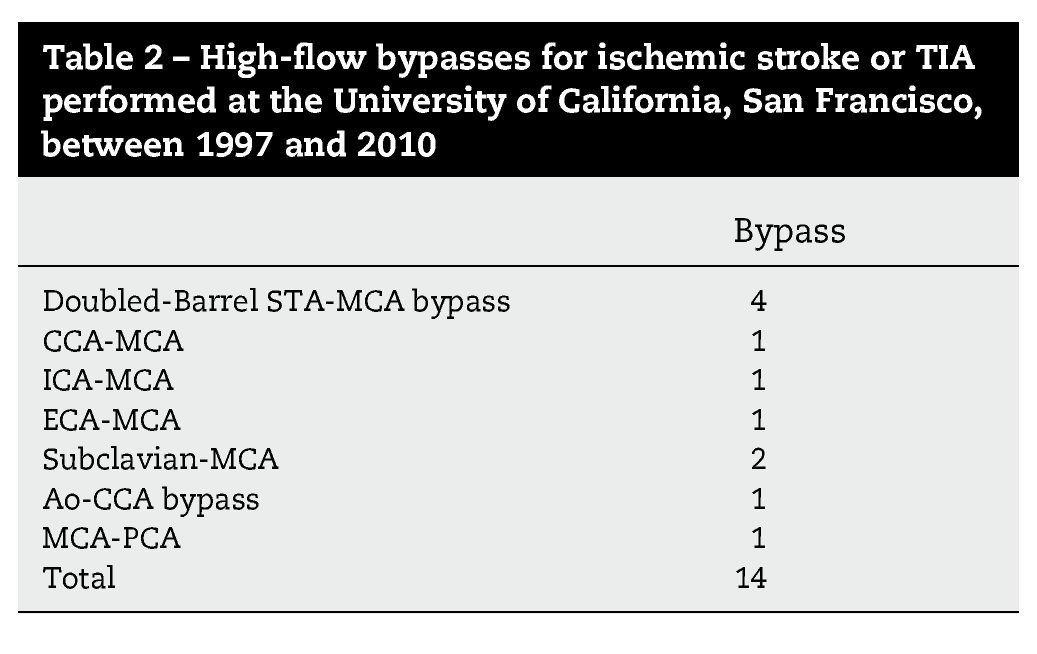
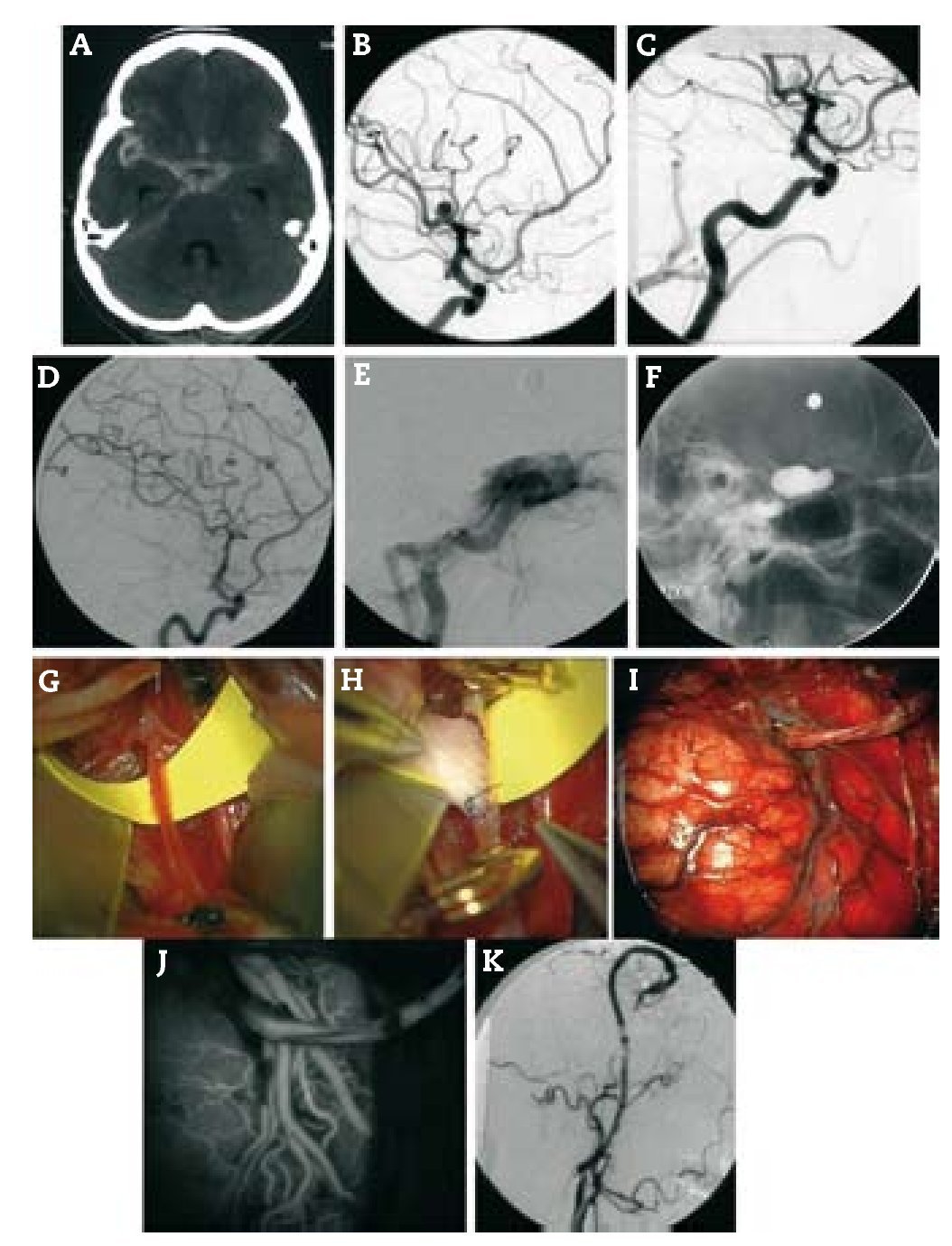
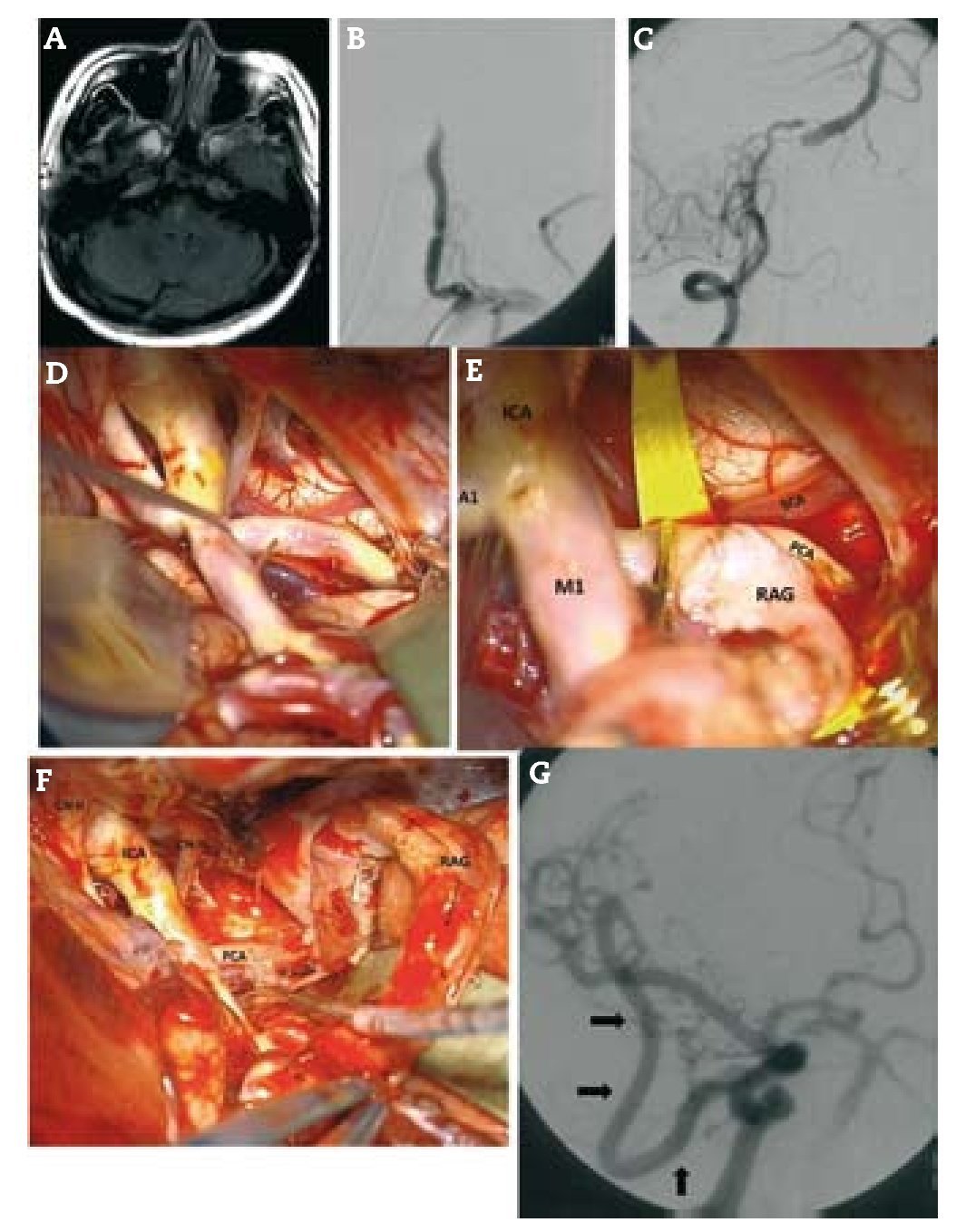
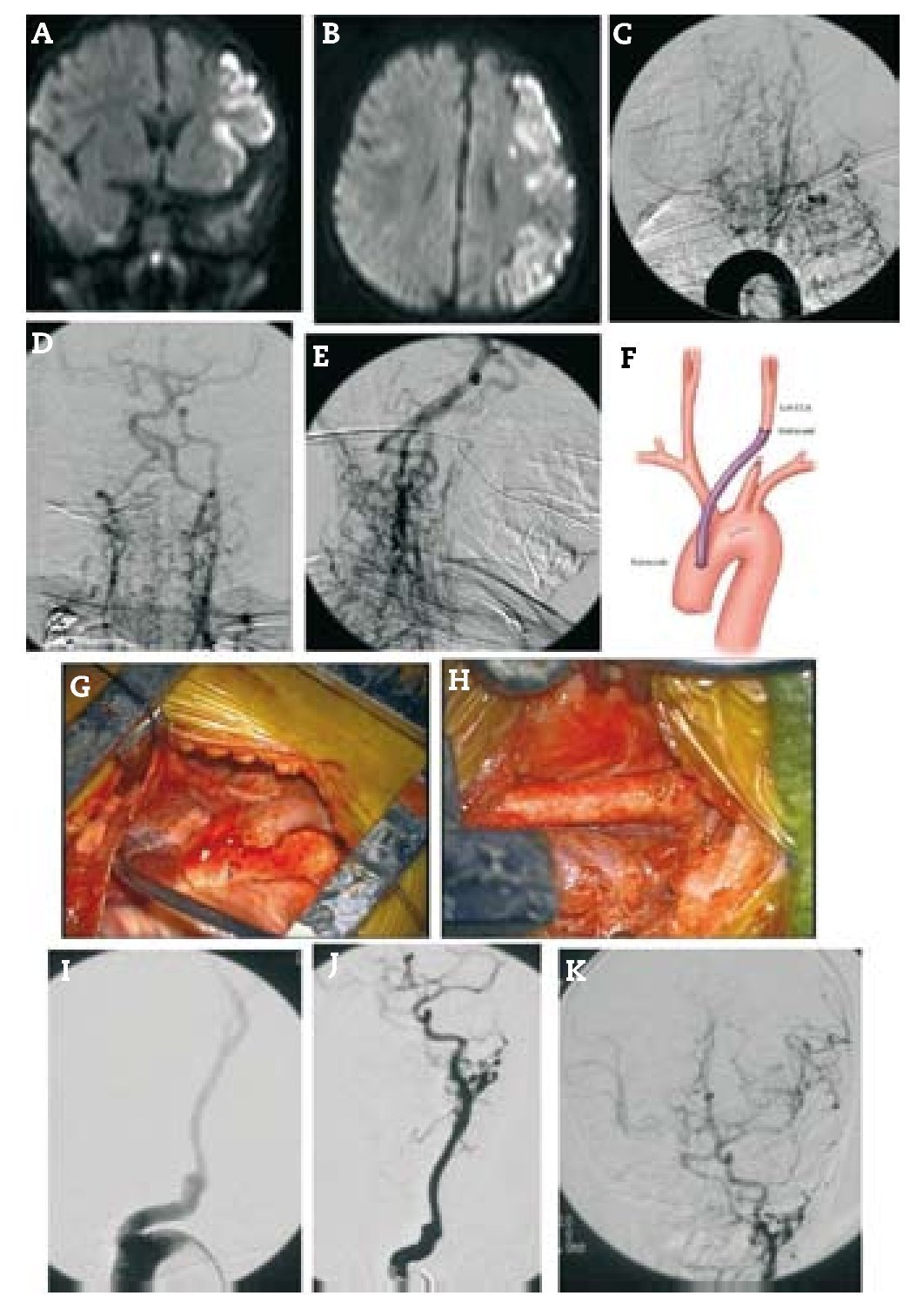
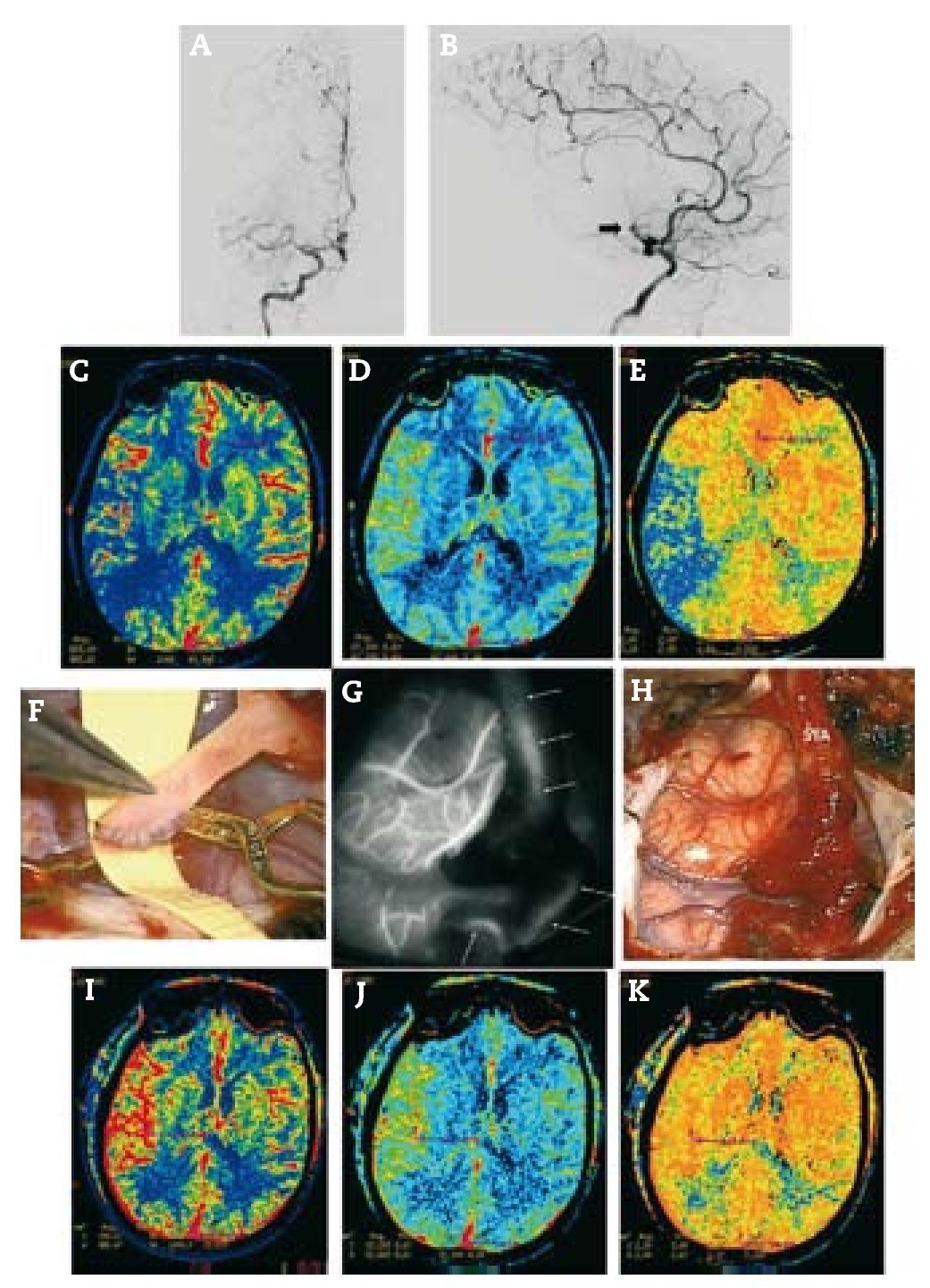
Resultados: Durante un período de 13 años, 152 bypasses fueron realizados por isquemia en 129 pacientes. Los diagnósticos específicos incluyeron: a) oclusión de la arteria carótida interna (ICA) (58 bypasses); b) oclusión de la arteria cerebral media y, raramente, la estenosis de alto grado de la misma (22 bypasses); c) ateroesclerosis esteno-oclusiva del sistema vertebrobasilar (2 bypasses); d) enfermedad de moya-moya (65 bypasses), y e) complicaciones isquémicas tras el tratamiento del aneurisma (5 bypasses). Ciento treinta y siete fueron bypasses convencionales de STA-MCA. Catorce pacientes tenían bypass de alto flujo que incluyó 4 «bouble-barrel» bypass de STA-MCA, 6 bypasses con injertos a la arteria carótida cervical, 2 de arteria subclavia a arteria cerebral media, un bypass de arteria cerebral media a arteria cerebral posterior, y un bypass aortocarotídeo. La tasa de permeabilidad de las anastomosis fue del 96,1%.
Conclusiones: La cirugía de bypass para la prevención del ictus isquémico es segura y se han desarrollado técnicas elegantes para ésta. Los pacientes con enfermedad atero-oclusiva, síntomas de isquemia e insuficiencia hemodinámica tienen un riesgo importante de accidente cerebrovascular si se manejan médicamente o no se tratan. Sin embargo, la intervención quirúrgica carece de evidencia científica que la respalde desde el reciente Carotid Occlusion Surgery Study (COSS). Los pacientes se ven atrapados en una situación difícil entre una historia natural pobre y una intervención quirúrgica sin evidencia. Los médicos deben individualizar el manejo de estos pacientes hasta que se publiquen datos adicionales o se desarrolle un mayor consenso.
Introduction: Although most ischemic strokes are thromboembolic in origin and their management is endovascular or medical, some are haemodynamic in origin and their management may be surgical. We reviewed bypass indications, patient selection and surgical techniques used in our current practice.
Methods: Extracranial-intracranial (EC-IC) bypass with superior temporal artery-to-middle cerebral artery (STA-MCA) bypass, high-flow interposition grafts and reconstructive techniques were used to treat patients with symptomatic ischemia.
Results: During a 13-year period, 152 bypasses were performed for ischemia in 129 patients. Specific diagnoses included: (1) internal carotid artery (ICA) occlusion (58 bypasses); (2) MCA occlusion and, rarely, high-grade MCA stenosis (22 bypasses); (3) vertebrobasilar atherosclerotic steno-occlusive disease (2 bypasses); (4) moyamoya disease (65 bypasses); and (5) ischemic complications after aneurysm treatment (5 bypasses). Of the 152 bypasses, 137 were conventional STA-MCA bypasses. Fourteen patients had high-flow bypasses that included 4 "double-barrel" STA-MCA bypasses, 6 bypasses with interposition grafts to the cervical carotid artery, 2 subclavian artery-to-MCA bypasses, 1 MCA-to-posterior cerebral artery (PCA) bypass and 1 aorto-carotid bypass. The bypass patency rate was 96.1%.
Conclusions: Bypass surgery for the prevention of ischemic stroke is safe and elegant techniques have been developed. Patients with athero-occlusive disease, ischemic symptoms and haemodynamic insufficiency have significant risk of stroke if managed medically or left untreated. However, surgical intervention lacks supporting evidence from the recent Carotid occlusion Surgery Study (COSS). Patients will be caught in a difficult position between a dismal natural history and an unproven surgical intervention. Clinicians must individualise their management until additional data are published or further consensus develops.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.