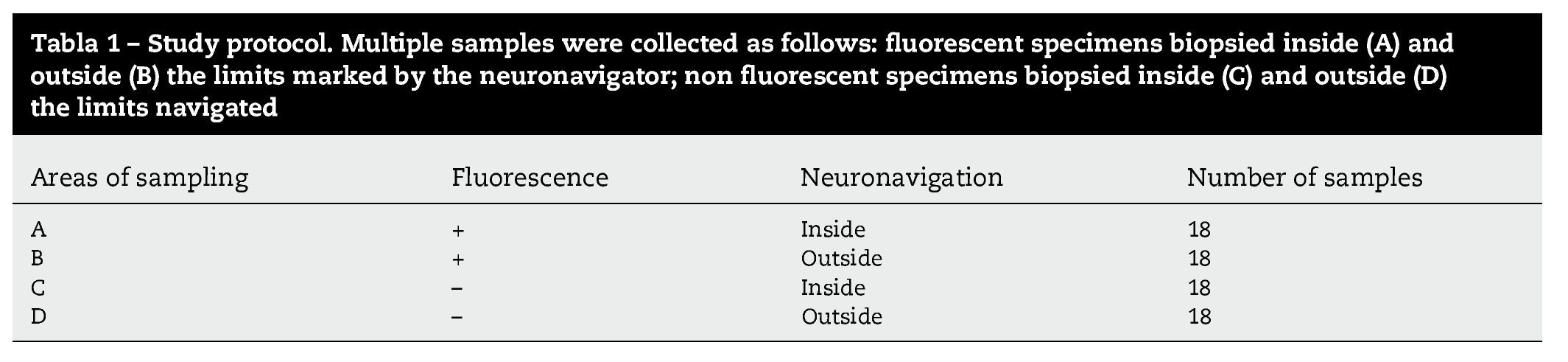
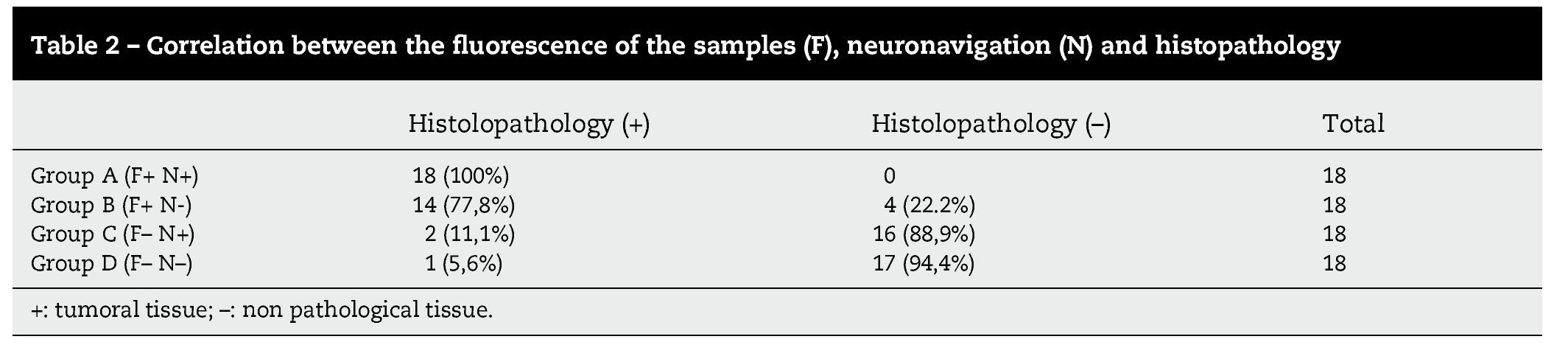
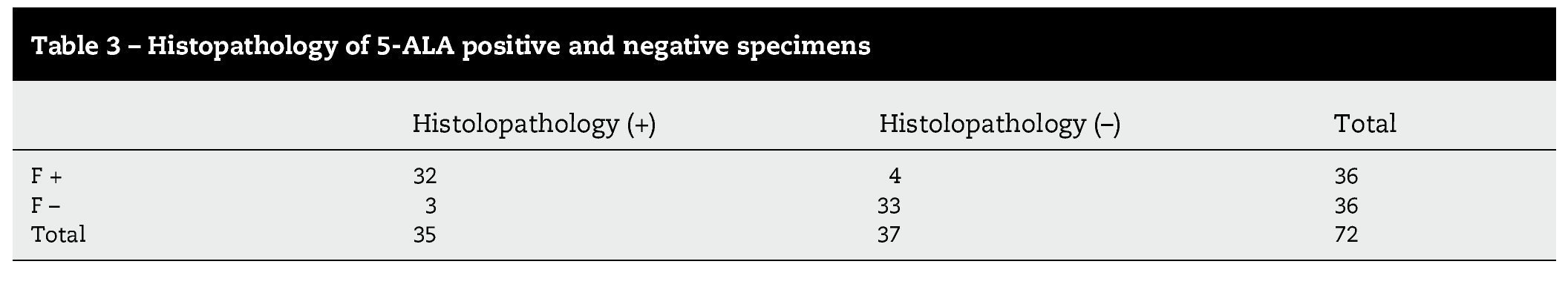
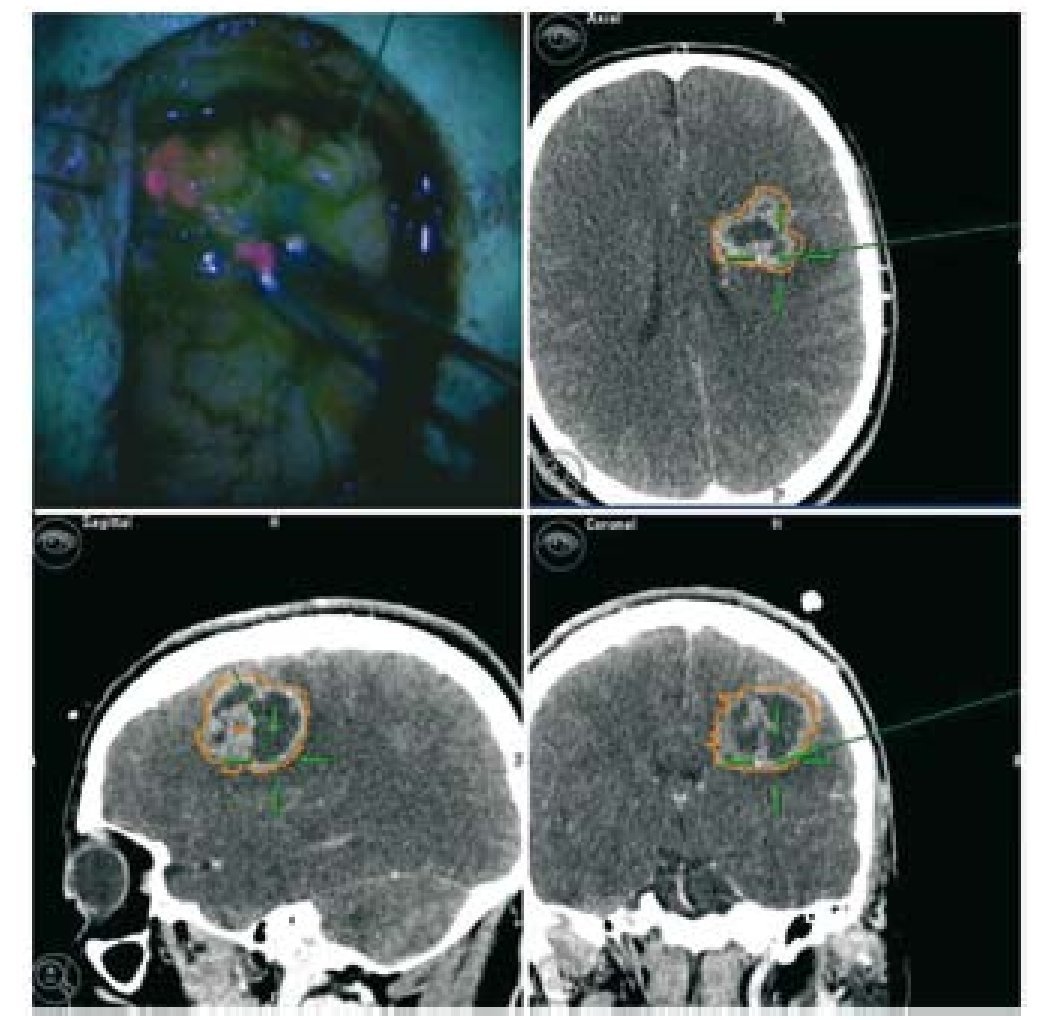
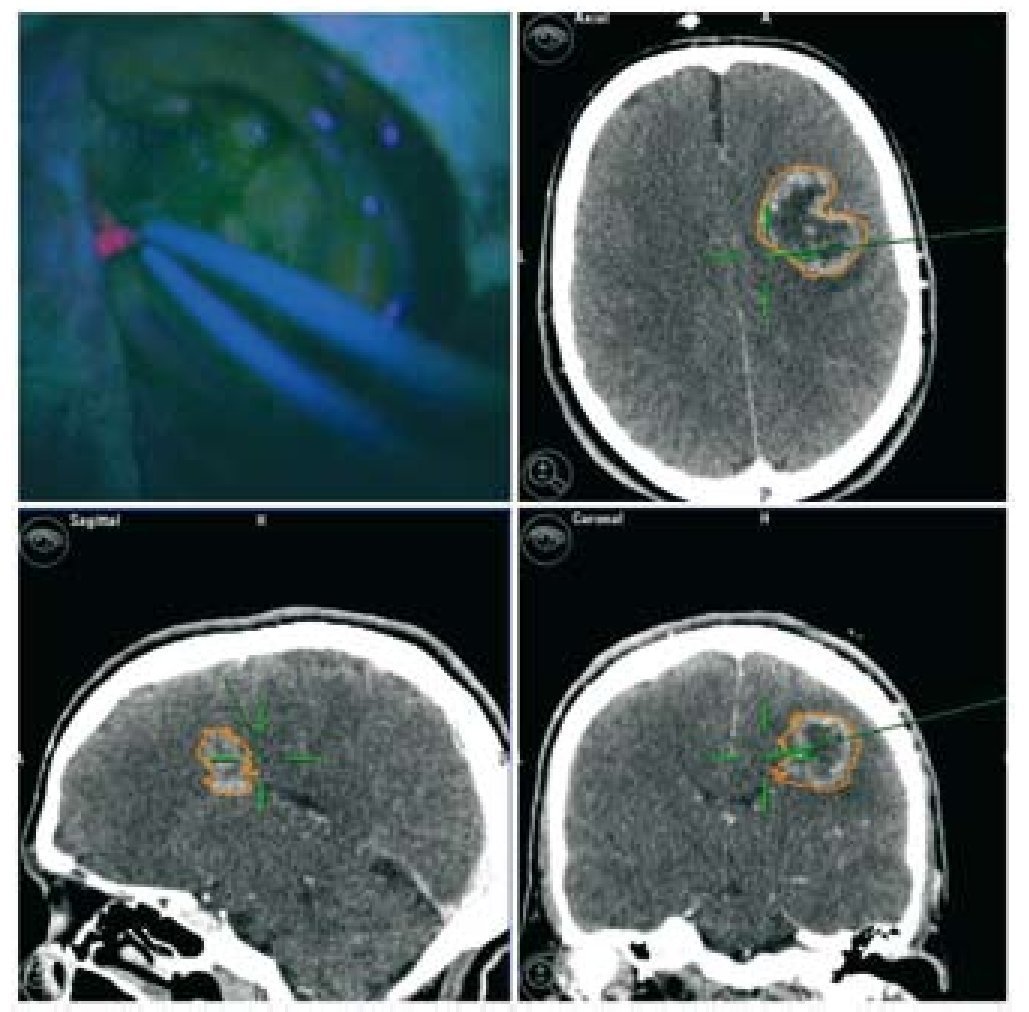
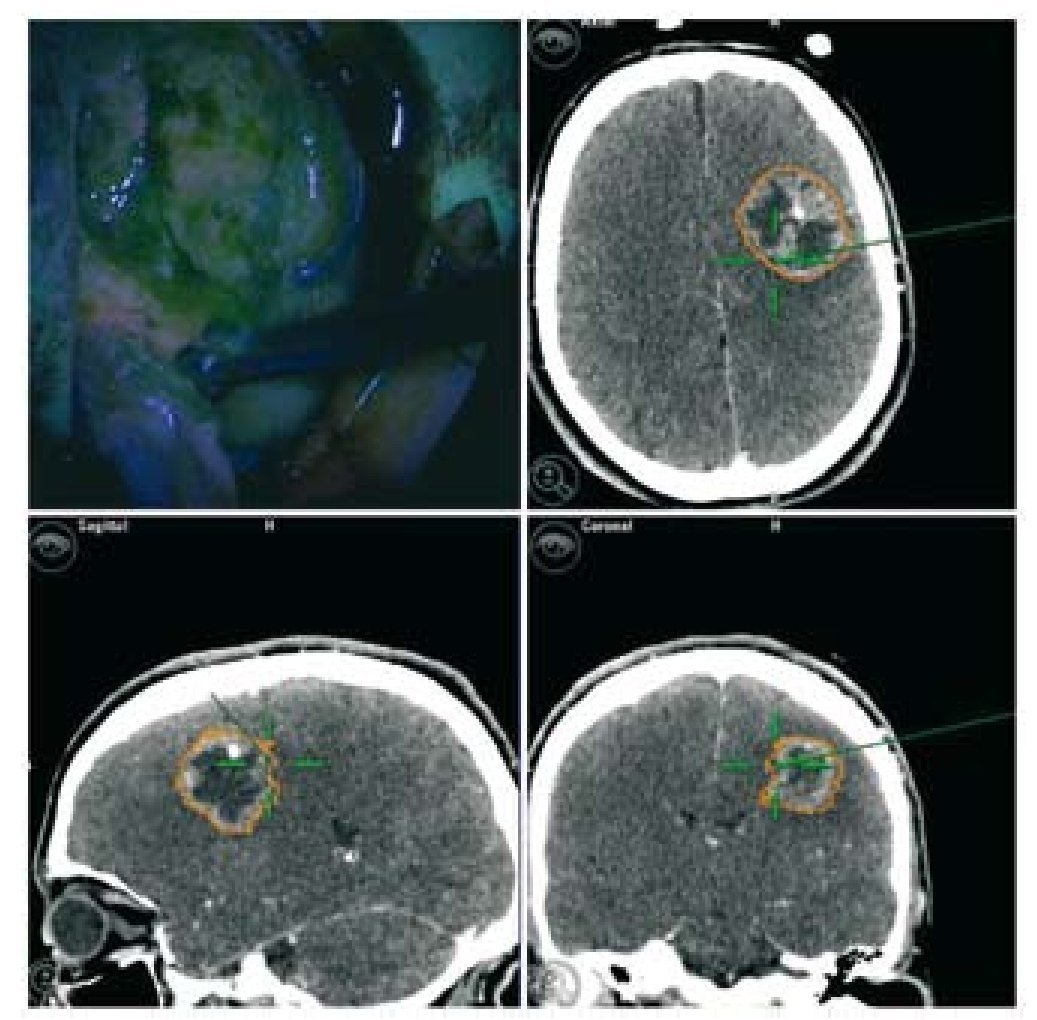
En la cirugía de gliomas de alto grado se utilizan diversas técnicas para lograr el máximo tratamiento citoreductivo y la conservación de las funciones neurológicas. Sin embargo, la efectividad de todos los métodos utilizados en solitario queda reducida por las limitaciones específicas de cada uno de estos métodos. Evaluamos la fiabilidad de una estrategia multimodal basada en el ácido 5-aminolevulínico (5-ALA) y la neuronavegación. Estudiamos de modo prospectivo a 18 pacientes con una zona sospechosa y no elocuente de gliomas malignos, susceptibles de resección completa. Se utilizó iluminación convencional hasta que la extirpación nos pareció completa. Se inspeccionó entonces sistemáticamente la cavidad con luz azul violeta para identificar cualquier tumor residual. En todos los casos se realizaron biopsias múltiples tanto del tejido fluorescente como del no fluorescente. Se etiquetó cada muestra conforme al emplazamiento de cada una de ellas (interior o exterior a los límites del neuronavegador). Las muestras fueron analizadas por un neuropatólogo, quien se atuvo a la clasificación intraoperatoria. Revisamos los resultados de ambos métodos, tanto de manera individual como combinada. El análisis individual mostró una mayor fiabilidad del 5-ALA en comparación a la neuronavegación. Sin embargo, se detectaron diversas muestras fluorescentes falso-negativas. Con el uso combinado de la fluorescencia y la neuroimagen, únicamente 1 muestra (negativa para el 5-ALA y la navegación) constituía tejido tumoral. En nuestra experiencia, la técnica combinada mostró una mejor sensibilidad, recomendándose para los casos de lesiones que impliquen zonas no elocuentes.
In high-grade glioma surgery, several techniques are used to achieve the maximum cytoreductive treatment preserving neurological functions. However, the effectiveness of all the methods used alone is reduced by specific limitations of each. We assessed the reliability of a multimodal strategy based on 5-aminolevulinic acid (5-ALA) and neuronavigation. We prospectively studied 18 patients with suspected, non eloquent-area malignant gliomas amenable for complete resection. Conventional illumination was used until the excision appeared complete. The cavity was then systematically inspected in violet-blue light to identify any residual tumour. Multiple biopsies of both fluorescent and non-fluorescent tissue were performed in all cases. Each specimen was labelled according to the sampling location (inside or outside the boundary set by the neuronavigator). The samples were analysed by a neuropathologist blinded to the intraoperative classification. We reviewed the results of both methods, either singly or in combination. Individual analysis showed higher 5-ALA reliability compared to neuronavigation. However, several false-negative fluorescent specimens were detected. With the combined use of fluorescence and neuroimaging, only 1 sample (negative for both 5-ALA and navigation) was tumoral tissue. In our experience, the combined approach showed the best sensitivity and it is recommended in cases of lesions involving non-eloquent areas.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.